Abstract
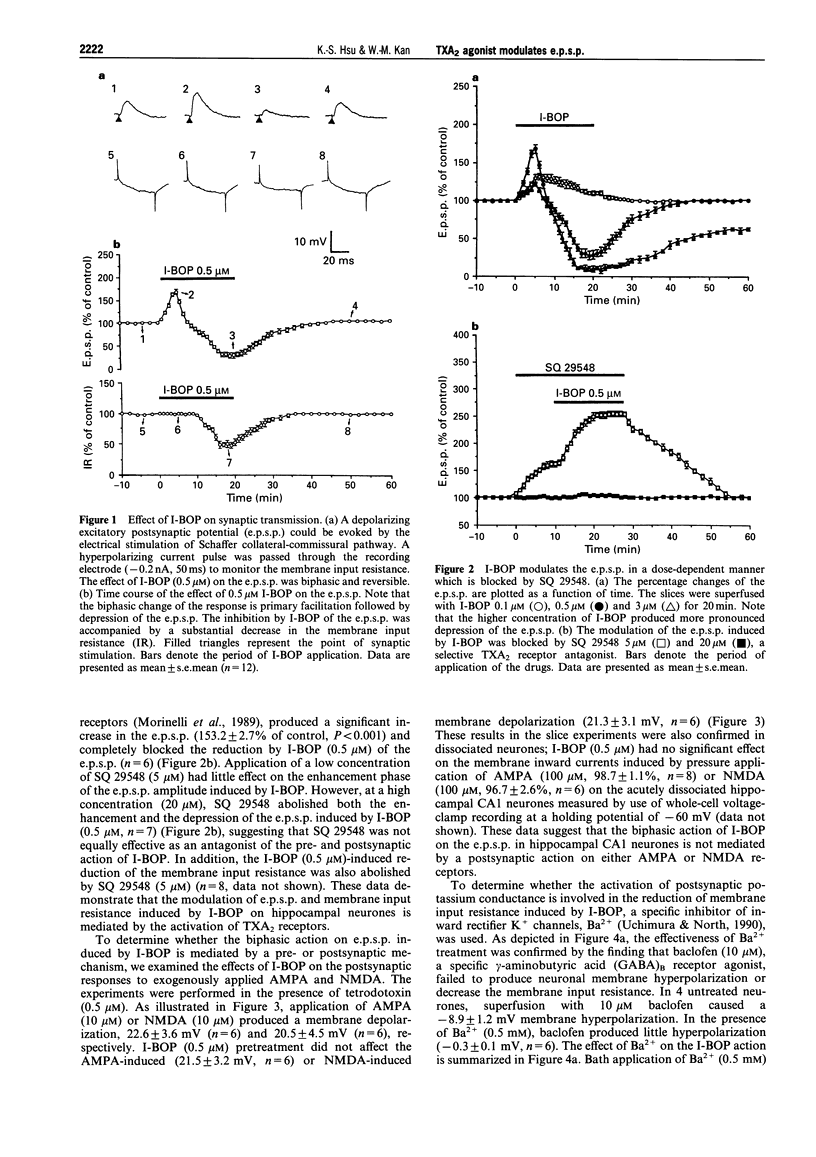
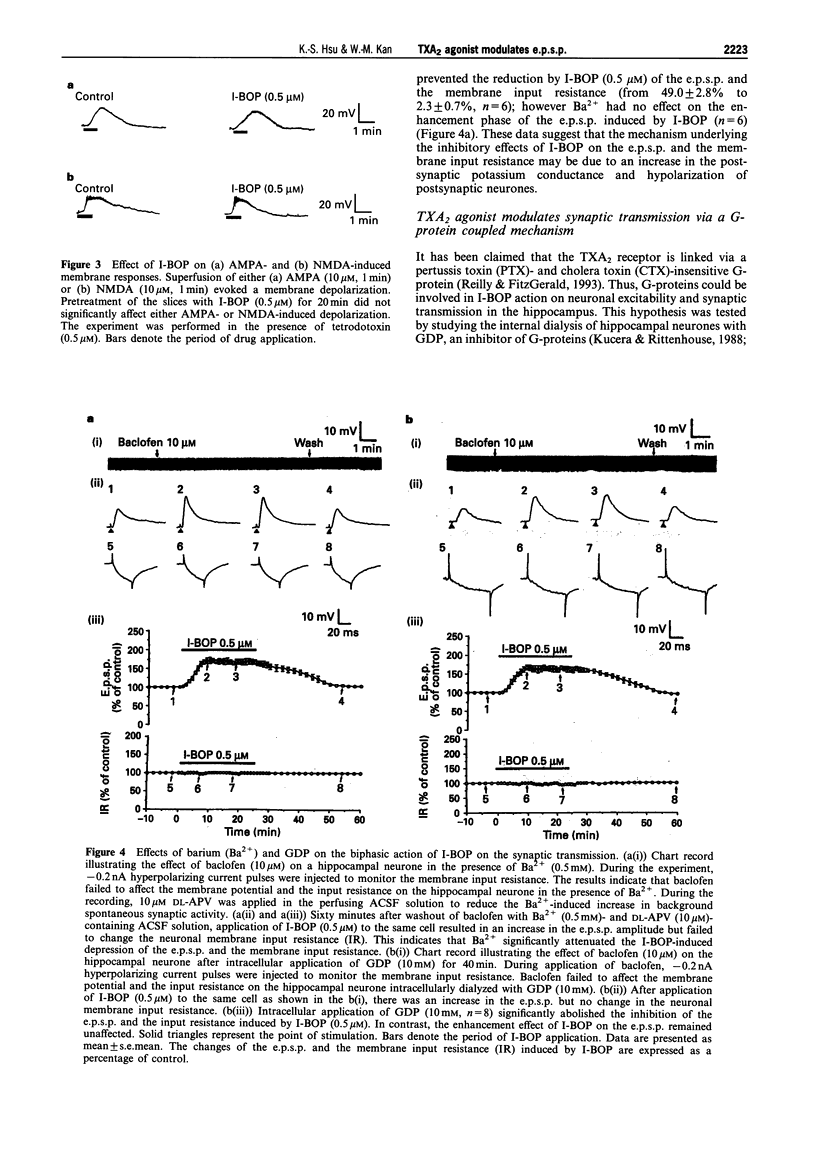
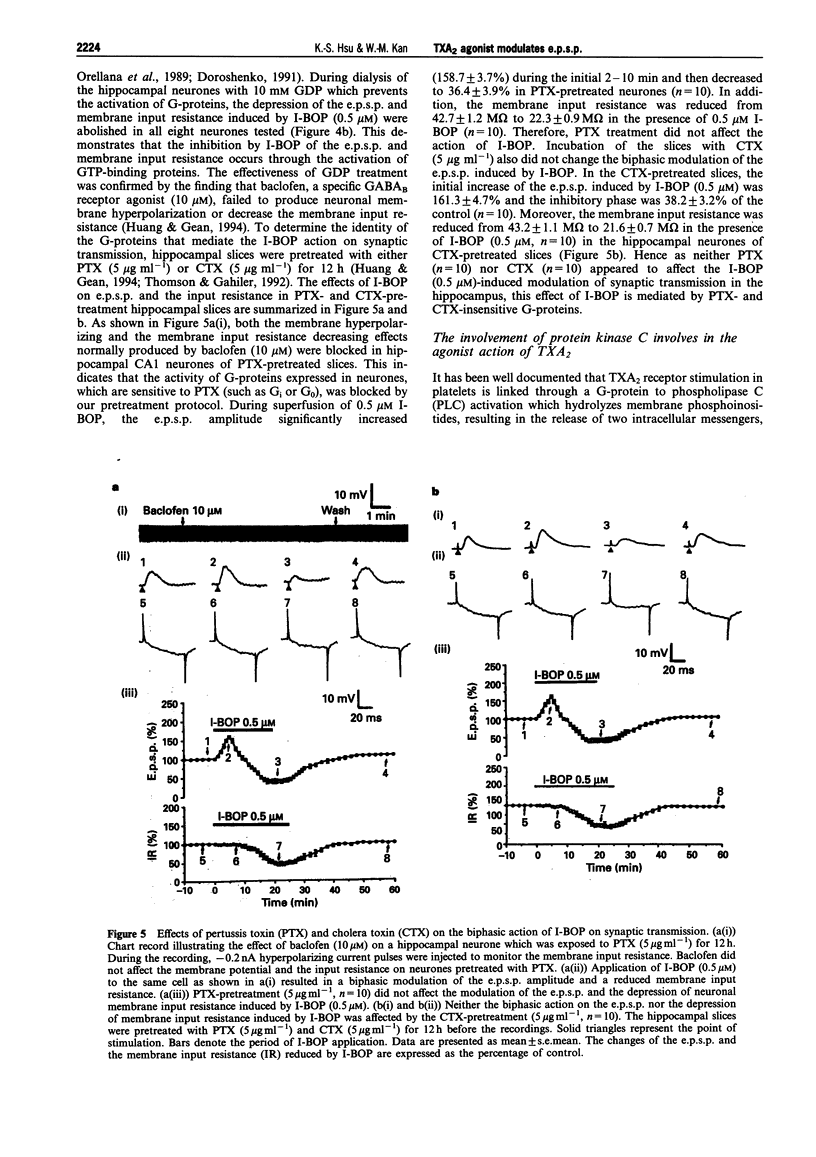
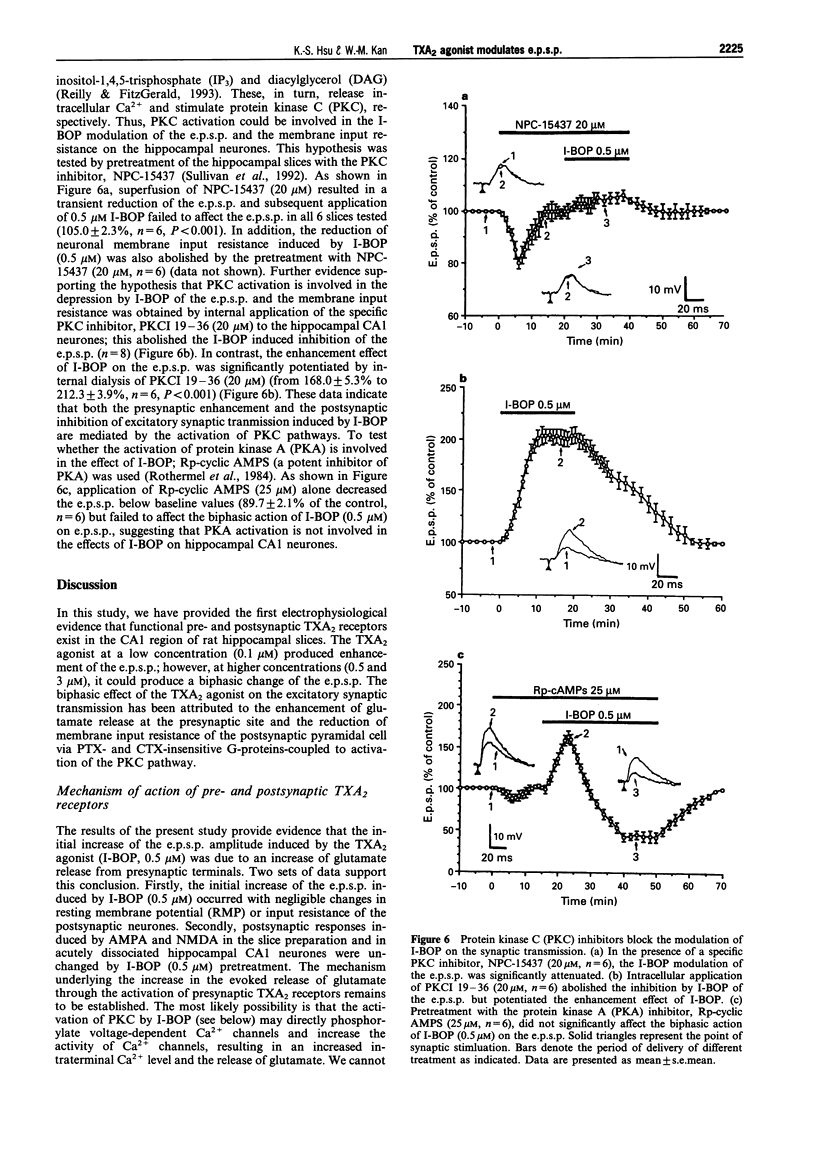
1. The effects of the selective thromboxane A2 (TXA2) receptor agonist I-BOP on neuronal excitability and synaptic transmission were studied in the CAl neurones of rat hippocampal slices by an intracellular recording technique. 2. Superfusion of I-BOP (0.5 microM) resulted in a biphasic change of the excitatory postsynaptic potential (e.p.s.p.), which was blocked by pretreatment with SQ 29548, a specific antagonist of TXA2 receptors. The inhibitory phase of I-BOP on the e.p.s.p. was accompanied by a decrease in neuronal membrane input resistance. 3. The sensitivity of postsynaptic neurones to glutamate receptor agonists, alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) or N-methyl-D-aspartate (NMDA), was unchanged by I-BOP (0.5 microM) pretreatment. 4. Bath application of Ba2+ (0.5 mM) prevented both the I-BOP-induced reduction of the neuronal membrane input resistance and the blockade of e.p.s.p. induced by I-BOP. 5. Intracellular dialysis of the hippocampal CA1 neurones with GDP (10 mM) significantly attenuated the I-BOP inhibition of e.p.s.p. and membrane input resistance. Incubation of the slices with either pertussis toxin (PTX, 5 micrograms ml-1 for 12 h) or cholera toxin (CTX, 5 micrograms ml-1 for 12 h) did not affect the biphasic action of I-BOP on the e.p.s.p. or the reduction of membrane input resistance induced by I-BOP. 6. Pretreatment of the slices with the protein kinase C (PKC) inhibitor, NPC-15437 (20 microM), abolished the biphasic modulation by I-BOP (0.5 microM) of the e.p.s.p. Intracellular application of a specific PKC inhibitor, PKCI 19-36 (20 microM), completely inhibited the I-BOP reduction of e.p.s.p. The specific cyclic AMP-dependent protein kinase (PKA) inhibitor, Rp-cyclic adenosine 3',5'-monophosphate (Rp-cyclic AMPS, 25 microM), had no effect on the I-BOP action. 7. In this study we have demonstrated, for the first time, the existence of functional TXA2 receptors in the hippocampus which mediate the effects of a TXA2 agonist on neuronal excitability and synaptic transmission. Activation of the presynaptic TXA2 receptors may stimulate the release of glutamate. Conversely, activation of postsynaptic TXA2 receptors leads to inhibition of synaptic transmission resulting from a decrease in the membrane input resistance of the neurones. The pre- and postsynaptic actions of the TXA2 agonist are both mediated by PTX- and CTX-insensitive G-protein-coupled activation of PKC pathways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Carlson K. E., Brass L. F., Manning D. R. Thrombin and phorbol esters cause the selective phosphorylation of a G protein other than Gi in human platelets. J Biol Chem. 1989 Aug 5;264(22):13298–13305. [PubMed] [Google Scholar]
- Cerne R., Jiang M., Randić M. Cyclic adenosine 3'5'-monophosphate potentiates excitatory amino acid and synaptic responses of rat spinal dorsal horn neurons. Brain Res. 1992 Nov 20;596(1-2):111–123. doi: 10.1016/0006-8993(92)91538-p. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Hsu C. Y., Hogan E. L., Halushka P. V., Linet O. I., Yatsu F. M. Thromboxane, prostacyclin, and leukotrienes in cerebral ischemia. Neurology. 1986 Apr;36(4):466–470. doi: 10.1212/wnl.36.4.466. [DOI] [PubMed] [Google Scholar]
- Doroshenko P. Second messengers mediating activation of chloride current by intracellular GTP gamma S in bovine chromaffin cells. J Physiol. 1991 May;436:725–738. doi: 10.1113/jphysiol.1991.sp018576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostmann W. R., Taylor S. S., Genieser H. G., Jastorff B., Døskeland S. O., Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3',5'-cyclic phosphorothioates. J Biol Chem. 1990 Jun 25;265(18):10484–10491. [PubMed] [Google Scholar]
- FitzGerald G. A. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol. 1991 Sep 3;68(7):11B–15B. doi: 10.1016/0002-9149(91)90379-y. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. J., Roy L., Catella F., FitzGerald G. A. Platelet activation in unstable coronary disease. N Engl J Med. 1986 Oct 16;315(16):983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- Frey U., Huang Y. Y., Kandel E. R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993 Jun 11;260(5114):1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Heemskerk J. W., Feijge M. A., Sage S. O., Walter U. Indirect regulation of Ca2+ entry by cAMP-dependent and cGMP-dependent protein kinases and phospholipase C in rat platelets. Eur J Biochem. 1994 Jul 15;223(2):543–551. doi: 10.1111/j.1432-1033.1994.tb19023.x. [DOI] [PubMed] [Google Scholar]
- Hsu K. S., Huang C. C., Gean P. W. Voltage- and use-dependent block by 1-methyl-4-phenylpyridinium ion (MPP+) of N-methyl-D-aspartate-activated currents in rat hippocampal neurons. Neurosci Lett. 1995 Apr 7;189(1):17–20. doi: 10.1016/0304-3940(95)11438-3. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Gean P. W. Paired-pulse depression of the N-methyl-D-aspartate receptor-mediated synaptic potentials in the amygdala. Br J Pharmacol. 1994 Nov;113(3):1029–1035. doi: 10.1111/j.1476-5381.1994.tb17096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera G. L., Rittenhouse S. E. Inhibition of GDP beta S of agonist-activated phospholipase C in human platelets requires cell permeabilization. Biochem Biophys Res Commun. 1988 May 31;153(1):417–421. doi: 10.1016/s0006-291x(88)81240-3. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Izumiyama M., Onodera H., Kurosawa A., Kogure K. Effect of a novel thromboxane A2 receptor antagonist, S-1452, on postischemic brain injury in rats. Stroke. 1993 Dec;24(12):2059–2065. doi: 10.1161/01.str.24.12.2059. [DOI] [PubMed] [Google Scholar]
- Morinelli T. A., Oatis J. E., Jr, Okwu A. K., Mais D. E., Mayeux P. R., Masuda A., Knapp D. R., Halushka P. V. Characterization of an 125I-labeled thromboxane A2/prostaglandin H2 receptor agonist. J Pharmacol Exp Ther. 1989 Nov;251(2):557–562. [PubMed] [Google Scholar]
- Nakahata N., Ishimoto H., Kurita M., Ohmori K., Takahashi A., Nakanishi H. The presence of thromboxane A2 receptors in cultured astrocytes from rabbit brain. Brain Res. 1992 Jun 26;583(1-2):100–104. doi: 10.1016/s0006-8993(10)80013-7. [DOI] [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Parfitt K. D., Madison D. V. Phorbol esters enhance synaptic transmission by a presynaptic, calcium-dependent mechanism in rat hippocampus. J Physiol. 1993 Nov;471:245–268. doi: 10.1113/jphysiol.1993.sp019900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce B., Murphy S., Jeremy J., Morrow C., Dandona P. ATP-evoked Ca2+ mobilisation and prostanoid release from astrocytes: P2-purinergic receptors linked to phosphoinositide hydrolysis. J Neurochem. 1989 Mar;52(3):971–977. doi: 10.1111/j.1471-4159.1989.tb02549.x. [DOI] [PubMed] [Google Scholar]
- Pickard J. D. Role of prostaglandins and arachidonic acid derivatives in the coupling of cerebral blood flow to cerebral metabolism. J Cereb Blood Flow Metab. 1981;1(4):361–384. doi: 10.1038/jcbfm.1981.41. [DOI] [PubMed] [Google Scholar]
- Pulsinelli W. A., Brierley J. B., Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982 May;11(5):491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Reilly M., Fitzgerald G. A. Cellular activation by thromboxane A2 and other eicosanoids. Eur Heart J. 1993 Dec;14 (Suppl K):88–93. [PubMed] [Google Scholar]
- Rothermel J. D., Jastorff B., Botelho L. H. Inhibition of glucagon-induced glycogenolysis in isolated rat hepatocytes by the Rp diastereomer of adenosine cyclic 3',5'-phosphorothioate. J Biol Chem. 1984 Jul 10;259(13):8151–8155. [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Shirahase H., Usui H., Kurahashi K., Fujiwara M., Fukui K. Endothelium-dependent contraction induced by nicotine in isolated canine basilar artery--possible involvement of a thromboxane A2 (TXA2) like substance. Life Sci. 1988;42(4):437–445. doi: 10.1016/0024-3205(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Shohami E., Jacobs T. P., Hallenbeck J. M., Feuerstein G. Increased thromboxane A2 and 5-HETE production following spinal cord ischemia in the rabbit. Prostaglandins Leukot Med. 1987 Jul;28(2):169–181. doi: 10.1016/0262-1746(87)90161-2. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Auer R. N., Siesjö B. K. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol. 1984;64(4):319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- Sullivan J. P., Connor J. R., Shearer B. G., Burch R. M. 2,6-Diamino-N-([1-(1-oxotridecyl)-2-piperidinyl] methyl)hexanamide (NPC 15437): a novel inhibitor of protein kinase C interacting at the regulatory domain. Mol Pharmacol. 1992 Jan;41(1):38–44. [PubMed] [Google Scholar]
- Sullivan J. P., Connor J. R., Tiffany C., Shearer B. G., Burch R. M. NPC 15437 interacts with the C1 domain of protein kinase C. An analysis using mutant PKC constructs. FEBS Lett. 1991 Jul 8;285(1):120–123. doi: 10.1016/0014-5793(91)80739-p. [DOI] [PubMed] [Google Scholar]
- Szatkowski M., Attwell D. Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends Neurosci. 1994 Sep;17(9):359–365. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Takahara K., Murray R., FitzGerald G. A., Fitzgerald D. J. The response to thromboxane A2 analogues in human platelets. Discrimination of two binding sites linked to distinct effector systems. J Biol Chem. 1990 Apr 25;265(12):6836–6844. [PubMed] [Google Scholar]
- Tegtmeier F., Weber C., Heister U., Haker I., Scheller D., Nikolov R., Höller M. Eicosanoids in rat brain during ischemia and reperfusion--correlation to DC depolarization. J Cereb Blood Flow Metab. 1990 May;10(3):358–364. doi: 10.1038/jcbfm.1990.65. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N., North R. A. Muscarine reduces inwardly rectifying potassium conductance in rat nucleus accumbens neurones. J Physiol. 1990 Mar;422:369–380. doi: 10.1113/jphysiol.1990.sp017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg E., Deshpande J. K., Wieloch T. Regional differences in arachidonic acid release in rat hippocampal CA1 and CA3 regions during cerebral ischemia. J Cereb Blood Flow Metab. 1987 Apr;7(2):189–192. doi: 10.1038/jcbfm.1987.43. [DOI] [PubMed] [Google Scholar]



