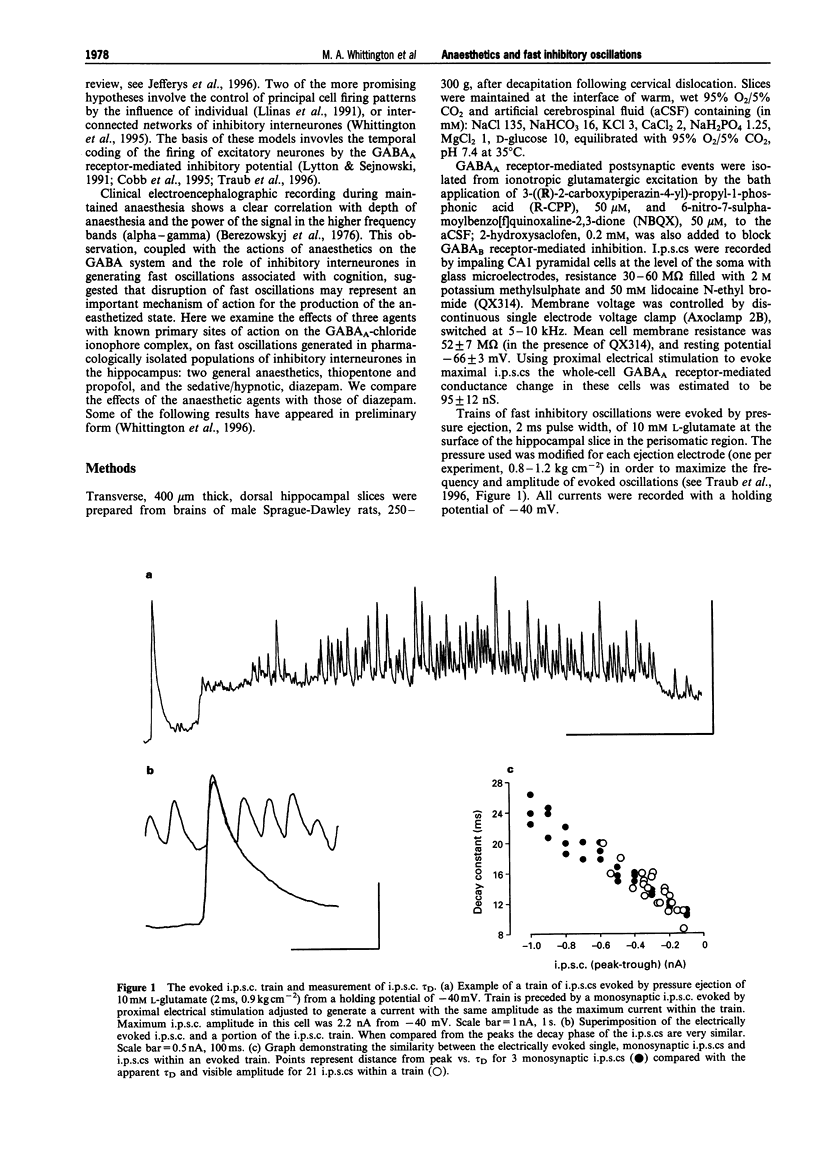
Abstract
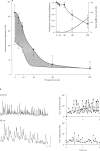
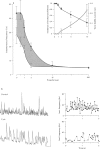
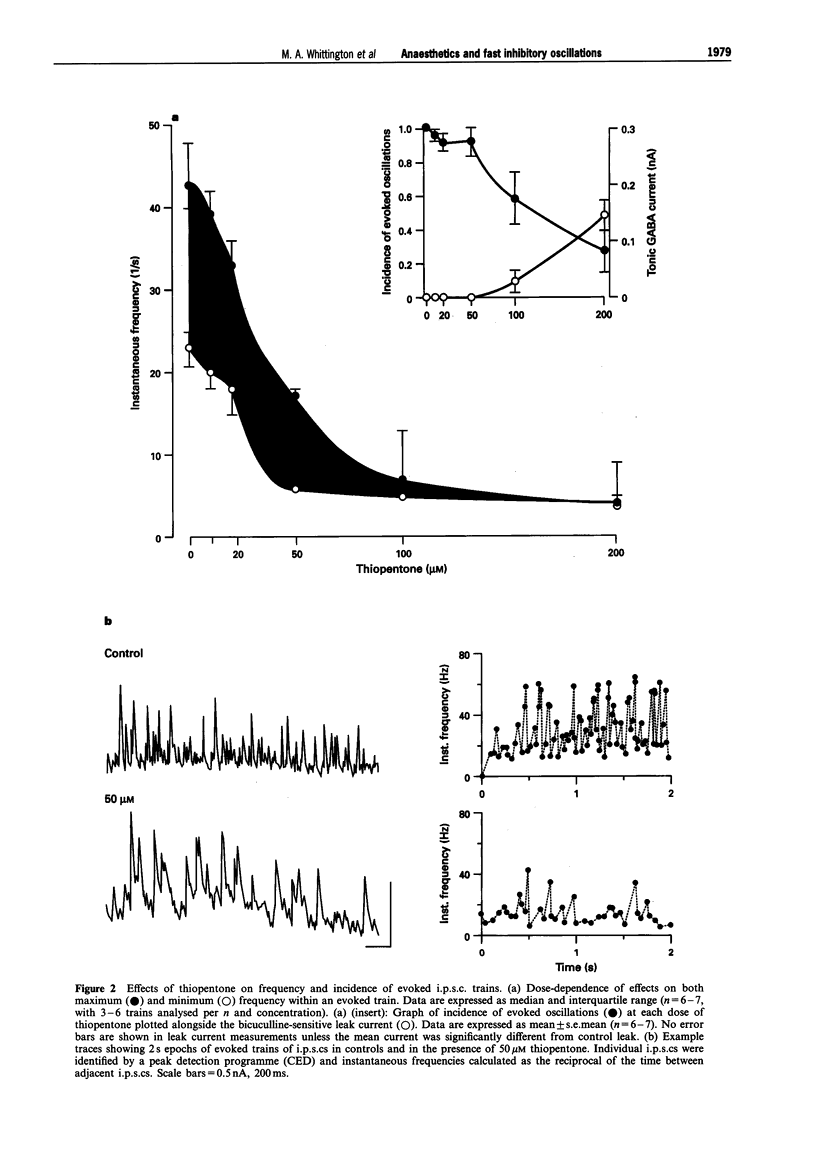
1. General anaesthetic agents prevent awareness of sensory input and subsequent recall of sensory events after administration. The mechanisms involved in higher sensory processing, including awareness and recall, are not fully elucidated. However, fast oscillations in neuronal activity in the 20-80 Hz (gamma) range have been strongly implicated. Here we have investigated the effects of two anaesthetic agents and a sedative/hypnotic drug on these oscillations. 2. Trains of fast oscillations, shown previously to be shaped by gamma-aminobutyric acid (GABAA) receptor activation, were evoked by pressure ejection of L-glutamate (10 nM) onto the perisomatic region of hippocampal area CAI in the presence of 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (R-CPP), 50 microM, 6-nitro-7-sulphamoylbenzo[f]quinoxaline-2,3-dione (NBQX), 50 microM and 2-hydroxysaclofen, 0.2 mM. 3. Thiopentone (10-200 microM) and propofol (0.5-10 microM) dose-dependently decreased both the maximum oscillation frequency, by approx. 90%, and the incidence of evoked rhythmic oscillations by approx. 60%. Diazepam (0.05-1 microM) decreased maximum oscillation frequency by about 40% but did not affect the incidence of evoked oscillations. 4. The similar effects of thiopentone and propofol were mediated by both a large (about 600%) increase in the decay constant (tau D) of GABAA receptor-mediated inhibitory postsynaptic currents (i.p.s.cs) and a bicuculline-sensitive leak current. The two drugs had differing effects on i.p.s.c. amplitude. Diazepam caused a small increase in tau D (about 170%) and did not alter leak currents at the doses used. 5. Effects of the anaesthetic agents were seen on the above measurements at similar concentrations to those estimated in the CNS during clinical and veterinary anaesthesia. We suggest that the effects on fast oscillations associated with cognition may contribute to the mechanism by which these agents produce general anaesthesia.
Full text
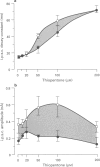
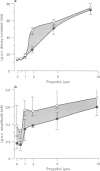
PDF
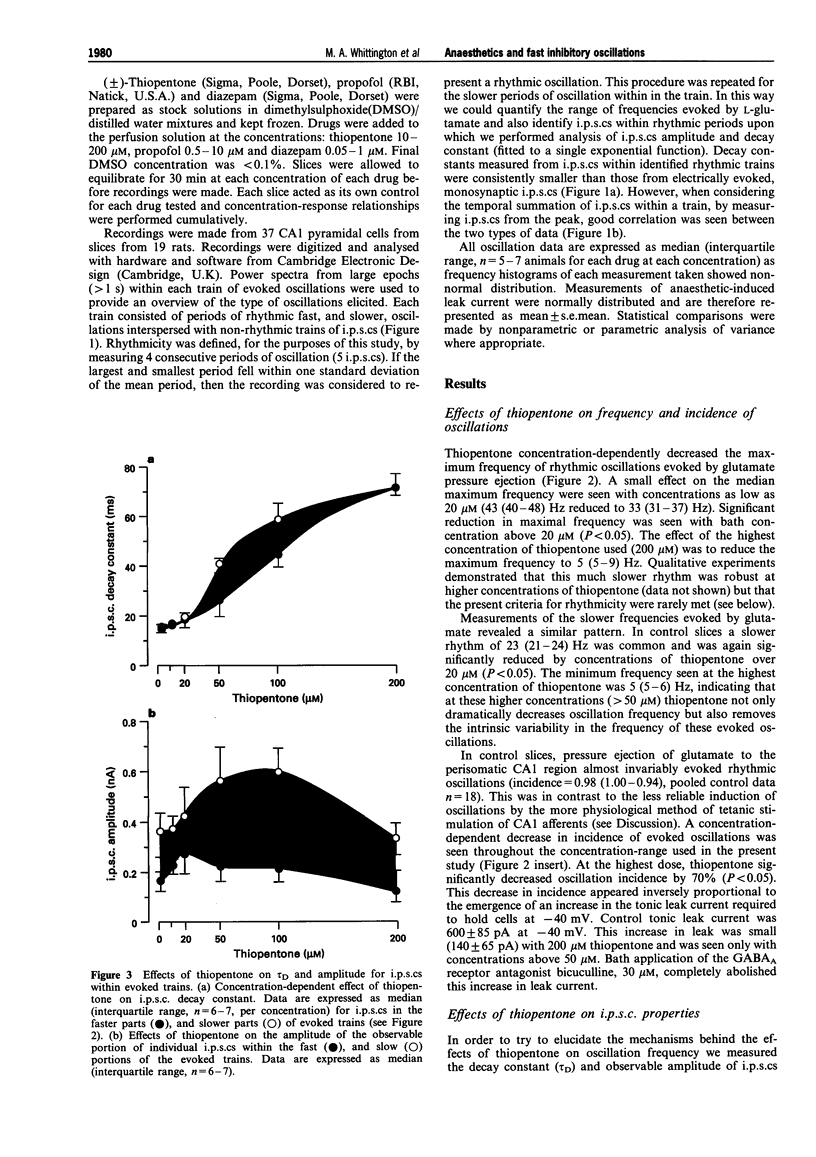
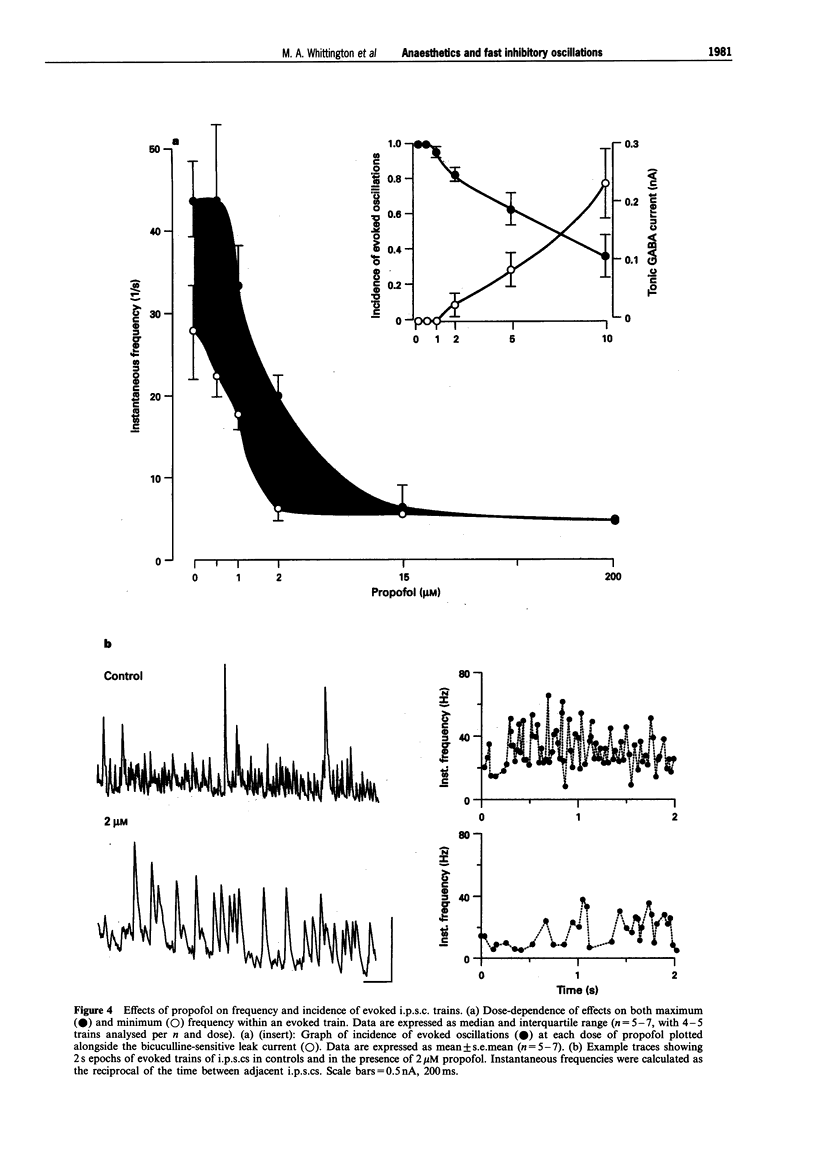
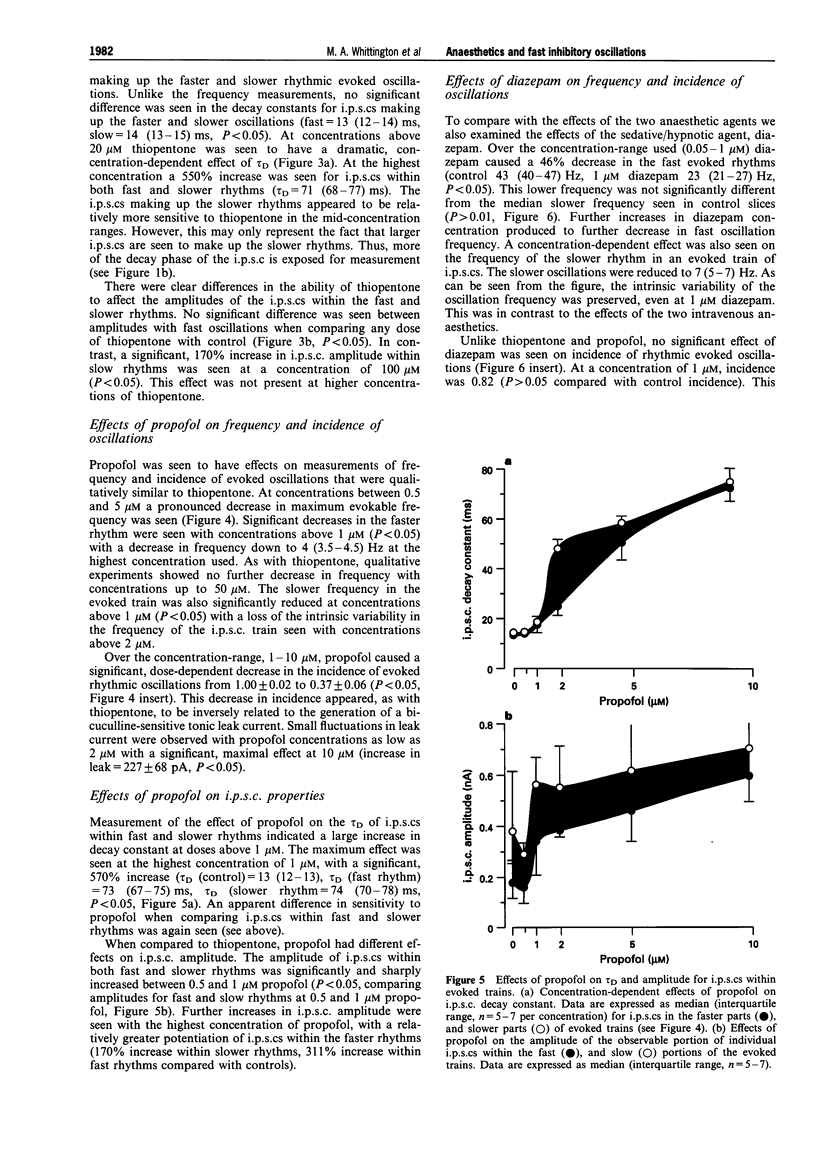
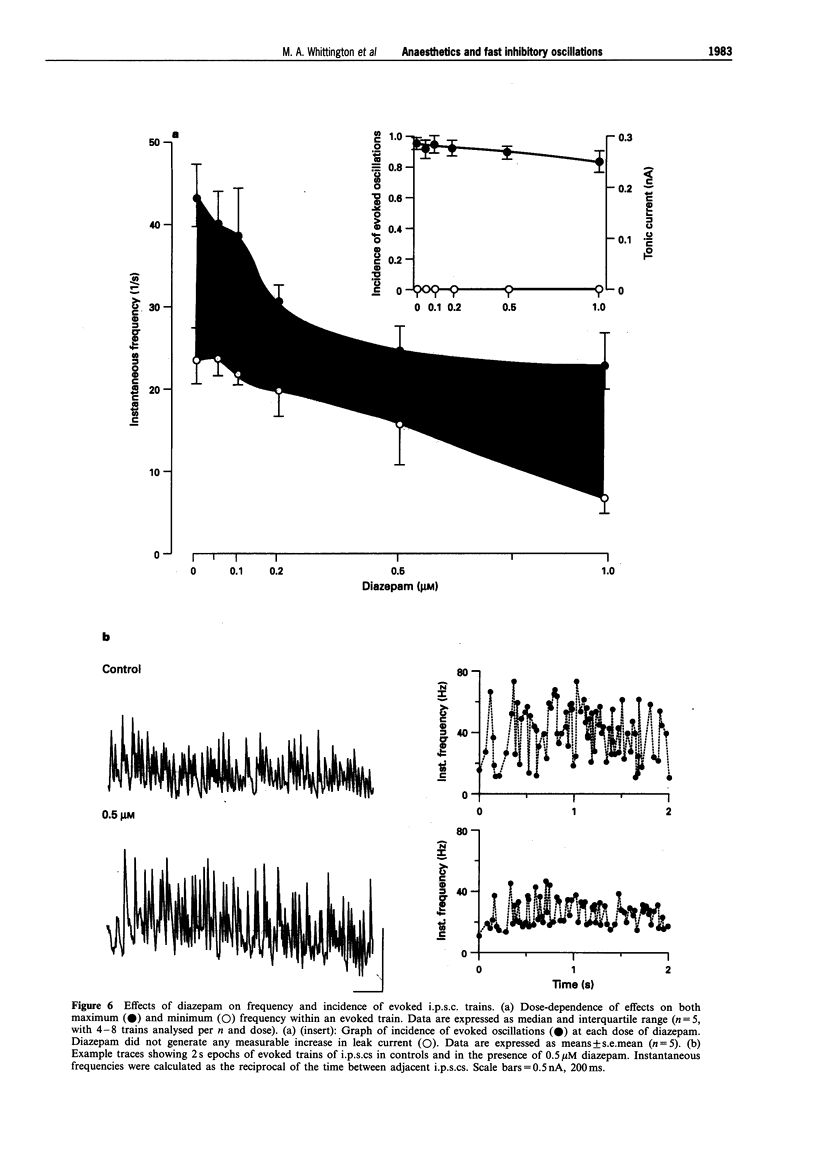
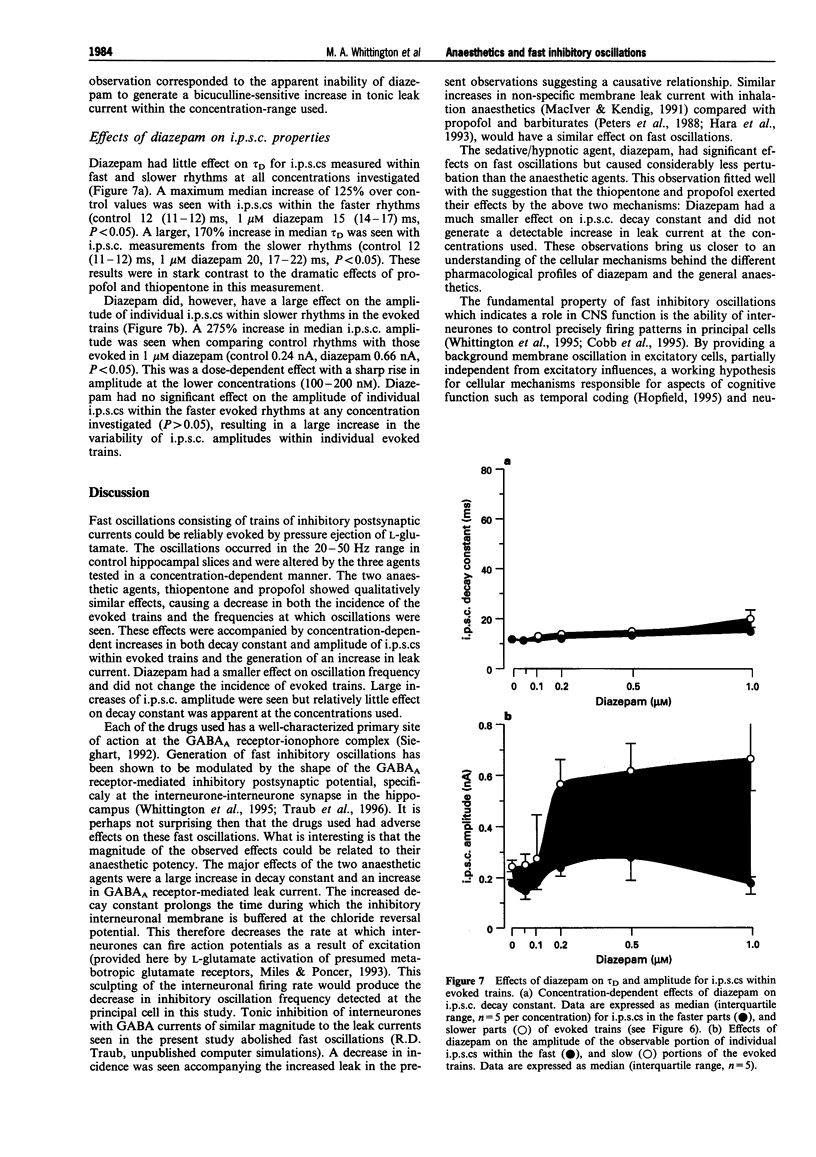


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel A. The G. L. Brown lecture. Adventures in anaesthesia. Exp Physiol. 1991 Jan;76(1):1–38. doi: 10.1113/expphysiol.1991.sp003471. [DOI] [PubMed] [Google Scholar]
- Berezowskyj J. L., McEwen J. A., Anderson G. B., Jenkins L. C. A study of anaesthesia depth by power spectral analysis of the electroencephalogram (EEG). Can Anaesth Soc J. 1976 Jan;23(1):1–8. doi: 10.1007/BF03004988. [DOI] [PubMed] [Google Scholar]
- Cobb S. R., Buhl E. H., Halasy K., Paulsen O., Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995 Nov 2;378(6552):75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cohen G. A., Doze V. A., Madison D. V. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992 Aug;9(2):325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- Doze V. A., Cohen G. A., Madison D. V. Synaptic localization of adrenergic disinhibition in the rat hippocampus. Neuron. 1991 Jun;6(6):889–900. doi: 10.1016/0896-6273(91)90229-s. [DOI] [PubMed] [Google Scholar]
- Engel A. K., Kreiter A. K., König P., Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6048–6052. doi: 10.1073/pnas.88.14.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. K., König P., Kreiter A. K., Schillen T. B., Singer W. Temporal coding in the visual cortex: new vistas on integration in the nervous system. Trends Neurosci. 1992 Jun;15(6):218–226. doi: 10.1016/0166-2236(92)90039-b. [DOI] [PubMed] [Google Scholar]
- Flohr H. An information processing theory of anaesthesia. Neuropsychologia. 1995 Sep;33(9):1169–1180. doi: 10.1016/0028-3932(95)00056-9. [DOI] [PubMed] [Google Scholar]
- Frank G. B., Ota M. Blockade of the reticulospinal inhibitory pathway by anaesthetic agents. Br J Pharmacol. 1971 Jul;42(3):328–342. doi: 10.1111/j.1476-5381.1971.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984 Aug 16;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985 Jul;85(3):675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R., Makeig S., Talmachoff P. J. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. M. Synchronous oscillations in neuronal systems: mechanisms and functions. J Comput Neurosci. 1994 Jun;1(1-2):11–38. doi: 10.1007/BF00962716. [DOI] [PubMed] [Google Scholar]
- Hales T. G., Lambert J. J. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991 Nov;104(3):619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Kai Y., Ikemoto Y. Propofol activates GABAA receptor-chloride ionophore complex in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology. 1993 Oct;79(4):781–788. doi: 10.1097/00000542-199310000-00021. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Vicini S., Barker J. L. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987 Feb;7(2):604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The actions of some general anaesthetics on the potassium current of the squid giant axon. J Physiol. 1986 Apr;373:311–327. doi: 10.1113/jphysiol.1986.sp016049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J. Pattern recognition computation using action potential timing for stimulus representation. Nature. 1995 Jul 6;376(6535):33–36. doi: 10.1038/376033a0. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Traub R. D., Whittington M. A. Neuronal networks for induced '40 Hz' rhythms. Trends Neurosci. 1996 May;19(5):202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Martin R. L., Redman S. J. Reduction by general anaesthetics of group Ia excitatory postsynaptic potentials and currents in the cat spinal cord. J Physiol. 1989 May;412:277–296. doi: 10.1113/jphysiol.1989.sp017615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little H. J. Effects of ketamine and of high pressure on the responses to gamma-aminobutyric acid of the rat superior cervical ganglion in vitro. Br J Pharmacol. 1982 Oct;77(2):209–216. doi: 10.1111/j.1476-5381.1982.tb09287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R., Grace A. A., Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton W. W., Sejnowski T. J. Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J Neurophysiol. 1991 Sep;66(3):1059–1079. doi: 10.1152/jn.1991.66.3.1059. [DOI] [PubMed] [Google Scholar]
- MacIver M. B., Kendig J. J. Anesthetic effects on resting membrane potential are voltage-dependent and agent-specific. Anesthesiology. 1991 Jan;74(1):83–88. doi: 10.1097/00000542-199101000-00014. [DOI] [PubMed] [Google Scholar]
- MacIver M. B., Tanelian D. L., Mody I. Two mechanisms for anesthetic-induced enhancement of GABAA-mediated neuronal inhibition. Ann N Y Acad Sci. 1991;625:91–96. doi: 10.1111/j.1749-6632.1991.tb33832.x. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Yoshida H. The benzodiazepine triazolam: direct and GABA depressant effects on cultured mouse spinal cord neurons. Eur J Pharmacol. 1987 Jul 2;139(1):53–60. doi: 10.1016/0014-2999(87)90496-1. [DOI] [PubMed] [Google Scholar]
- Miles R., Poncer J. C. Metabotropic glutamate receptors mediate a post-tetanic excitation of guinea-pig hippocampal inhibitory neurones. J Physiol. 1993 Apr;463:461–473. doi: 10.1113/jphysiol.1993.sp019605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. A., Kirkness E. F., Callachan H., Lambert J. J., Turner A. J. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br J Pharmacol. 1988 Aug;94(4):1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plourde G., Picton T. W. Human auditory steady-state response during general anesthesia. Anesth Analg. 1990 Nov;71(5):460–468. doi: 10.1213/00000539-199011000-00002. [DOI] [PubMed] [Google Scholar]
- Sieghart W. GABAA receptors: ligand-gated Cl- ion channels modulated by multiple drug-binding sites. Trends Pharmacol Sci. 1992 Dec;13(12):446–450. doi: 10.1016/0165-6147(92)90142-s. [DOI] [PubMed] [Google Scholar]
- Singer W., Gray C. M. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Tanelian D. L., Kosek P., Mody I., MacIver M. B. The role of the GABAA receptor/chloride channel complex in anesthesia. Anesthesiology. 1993 Apr;78(4):757–776. doi: 10.1097/00000542-199304000-00020. [DOI] [PubMed] [Google Scholar]
- Whittington M. A., Traub R. D., Jefferys J. G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995 Feb 16;373(6515):612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Woods D. L., Hillyard S. A., Courchesne E., Galambos R. Electrophysiological signs of split-second decision-making. Science. 1980 Feb 8;207(4431):655–657. doi: 10.1126/science.7352278. [DOI] [PubMed] [Google Scholar]
- von der Malsburg C., Schneider W. A neural cocktail-party processor. Biol Cybern. 1986;54(1):29–40. doi: 10.1007/BF00337113. [DOI] [PubMed] [Google Scholar]