Abstract
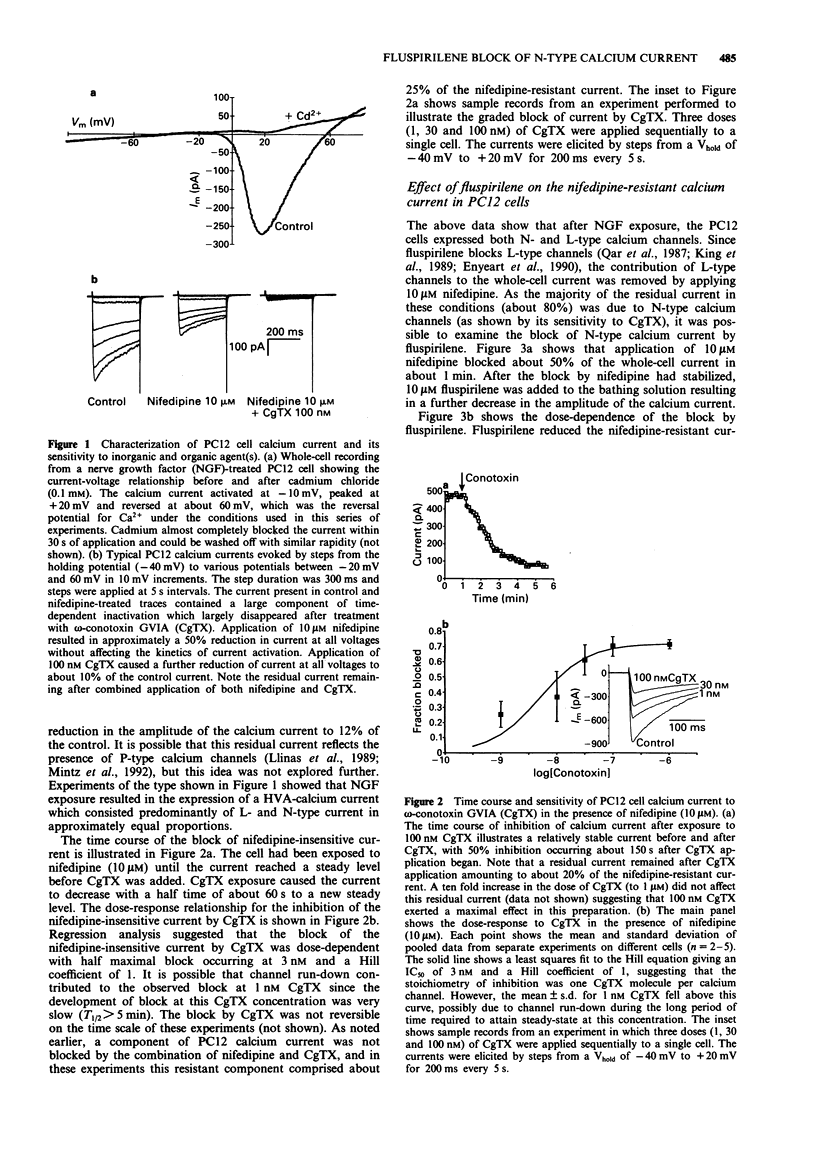
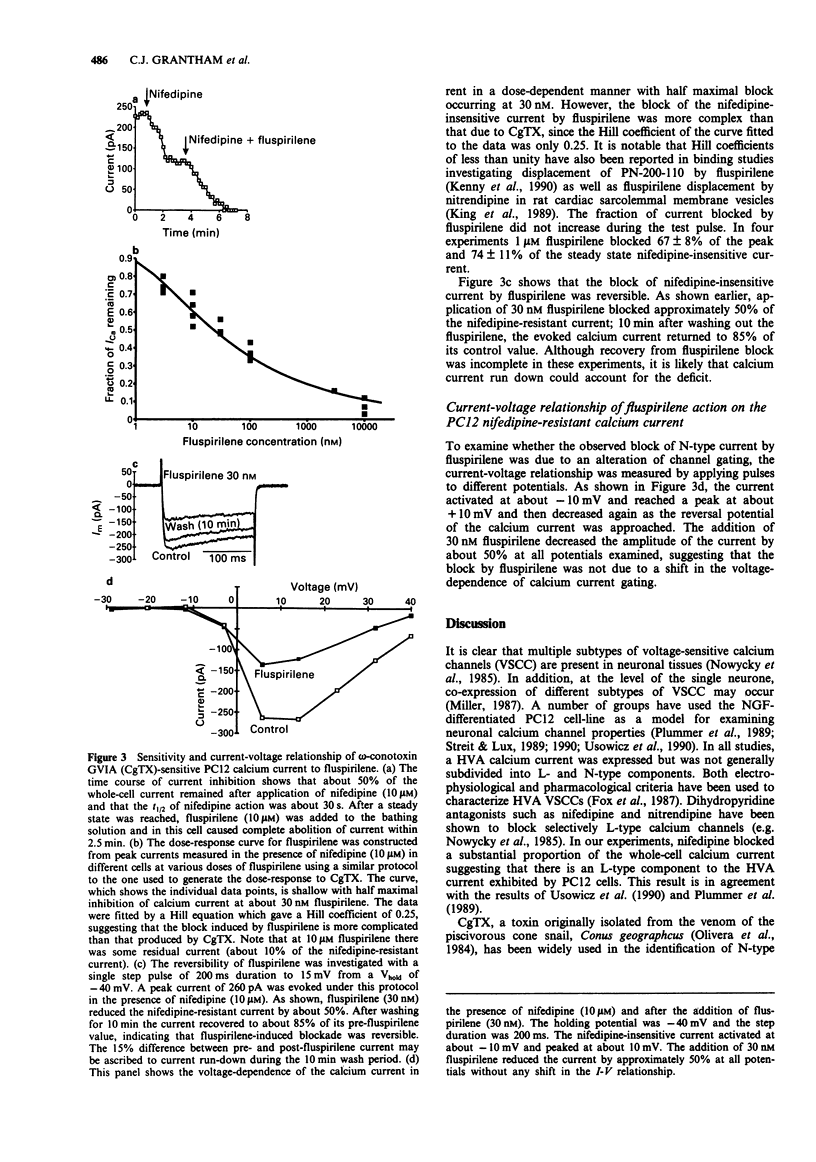
1. High voltage-activated calcium currents were recorded in nerve growth factor (NGF)-differentiated PC12 cells with the whole-cell patch clamp technique. After exposure to NGF for 3-10 days the PC12 cells developed neurone-like processes and calcium currents which were pharmacologically separable into L- and N-types (defined by sensitivity to nifedipine and omega-conotoxin GVIA respectively). 2. After blocking the L-type calcium channels with nifedipine (10 microM), omega-conotoxin GVIA blocked approximately 85% of the remaining calcium current with an IC50 of 3 nM and a Hill coefficient of 1. The block by conotoxin GVIA was irreversible on the time scale of these experiments. These results suggested that the majority of the nifedipine-insensitive calcium current was N-type. 3. Fluspirilene, a substituted diphenylbutylpiperidine with potent neuroleptic properties, reversibly inhibited the N-type component in a dose-dependent manner with an IC50 of 30 nM. The Hill coefficient of the block was 0.25. The fraction of current blocked was the same at all test potentials examined (-30 to +40 mV). 4. These data indicate that the neuroleptic properties of fluspirilene may be due, at least in part, to an inhibition of neuronal N-type calcium channels. This finding raises the possibility that modulation of N-type calcium channel activity by drugs derived from substituted diphenylbutylpiperidines may provide a novel way of altering neurotransmitter release and hence brain function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doherty P., Mann D. A., Walsh F. S. Comparison of the effects of NGF, activators of protein kinase C, and a calcium ionophore on the expression of Thy-1 and N-CAM in PC12 cell cultures. J Cell Biol. 1988 Jul;107(1):333–340. doi: 10.1083/jcb.107.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante W., Sen A. K., Sunahara F. A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988 Jun;94(2):463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart J. J., Biagi B. A., Day R. N., Sheu S. S., Maurer R. A. Blockade of low and high threshold Ca2+ channels by diphenylbutylpiperidine antipsychotics linked to inhibition of prolactin gene expression. J Biol Chem. 1990 Sep 25;265(27):16373–16379. [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Onodera H., Kogure K., Akaike N. Time-dependent expression of Na and Ca channels in PC12 cells by nerve growth factor and cAMP. Neurosci Res. 1993 Feb;16(2):143–147. doi: 10.1016/0168-0102(93)90081-z. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Fosset M., Romey G., Laduron P., Lazdunski M. Neuroleptics of the diphenylbutylpiperidine series are potent calcium channel inhibitors. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7513–7517. doi: 10.1073/pnas.83.19.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo G., Gottesmann C., Bidard J. N., Lazdunski M. Ca2+ channel blockers prevent seizures induced by a class of K+ channel inhibitors. Eur J Pharmacol. 1989 Jan 24;160(1):173–177. doi: 10.1016/0014-2999(89)90669-9. [DOI] [PubMed] [Google Scholar]
- Garber S. S., Hoshi T., Aldrich R. W. Regulation of ionic currents in pheochromocytoma cells by nerve growth factor and dexamethasone. J Neurosci. 1989 Nov;9(11):3976–3987. doi: 10.1523/JNEUROSCI.09-11-03976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. J., Murphy K. M., Reynolds I. J., Snyder S. H. Antischizophrenic drugs of the diphenylbutylpiperidine type act as calcium channel antagonists. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5122–5125. doi: 10.1073/pnas.80.16.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas S., Beckmann H. Pimozide versus Haloperidol in acute schizophrenia. A double blind controlled study. Pharmacopsychiatria. 1982 Mar;15(2):70–74. doi: 10.1055/s-2007-1019512. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hassel P. Experimental comparison of low doses of 1.5 mg fluspirilene and bromazepam in out-patients with psychovegetative disturbances. Pharmacopsychiatry. 1985 Sep;18(5):297–302. doi: 10.1055/s-2007-1017384. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Kenny B. A., Fraser S., Kilpatrick A. T., Spedding M. Selective antagonism of calcium channel activators by fluspirilene. Br J Pharmacol. 1990 Jun;100(2):211–216. doi: 10.1111/j.1476-5381.1990.tb15784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V. F., Garcia M. L., Shevell J. L., Slaughter R. S., Kaczorowski G. J. Substituted diphenylbutylpiperidines bind to a unique high affinity site on the L-type calcium channel. Evidence for a fourth site in the cardiac calcium entry blocker receptor complex. J Biol Chem. 1989 Apr 5;264(10):5633–5641. [PubMed] [Google Scholar]
- Lapierre Y. D. A controlled study of penfluridol in the treatment of chronic schizophrenia. Am J Psychiatry. 1978 Aug;135(8):956–959. doi: 10.1176/ajp.135.8.956. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., McIntosh J. M., Cruz L. J., Luque F. A., Gray W. R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984 Oct 23;23(22):5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Qar J., Galizzi J. P., Fosset M., Lazdunski M. Receptors for diphenylbutylpiperidine neuroleptics in brain, cardiac, and smooth muscle membranes. Relationship with receptors for 1,4-dihydropyridines and phenylalkylamines and with Ca2+ channel blockade. Eur J Pharmacol. 1987 Sep 11;141(2):261–268. doi: 10.1016/0014-2999(87)90271-8. [DOI] [PubMed] [Google Scholar]
- Quirion R., Lafaille F., Nair N. P. Comparative potencies of calcium channel antagonists and antischizophrenic drugs on central and peripheral calcium channel binding sites. J Pharm Pharmacol. 1985 Jun;37(6):437–440. doi: 10.1111/j.2042-7158.1985.tb03033.x. [DOI] [PubMed] [Google Scholar]
- Rudy B., Kirschenbaum B., Rukenstein A., Greene L. A. Nerve growth factor increases the number of functional Na channels and induces TTX-resistant Na channels in PC12 pheochromocytoma cells. J Neurosci. 1987 Jun;7(6):1613–1625. doi: 10.1523/JNEUROSCI.07-06-01613.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Sher E., Clementi F. Omega-conotoxin-sensitive voltage-operated calcium channels in vertebrate cells. Neuroscience. 1991;42(2):301–307. doi: 10.1016/0306-4522(91)90376-y. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Calcium current inactivation during nerve-growth-factor-induced differentiation of PC12 cells. Pflugers Arch. 1990 Jun;416(4):368–374. doi: 10.1007/BF00370742. [DOI] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Distribution of calcium currents in sprouting PC12 cells. J Neurosci. 1989 Dec;9(12):4190–4199. doi: 10.1523/JNEUROSCI.09-12-04190.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Voltage dependent calcium currents in PC12 growth cones and cells during NGF-induced cell growth. Pflugers Arch. 1987 May;408(6):634–641. doi: 10.1007/BF00581167. [DOI] [PubMed] [Google Scholar]
- Usowicz M. M., Porzig H., Becker C., Reuter H. Differential expression by nerve growth factor of two types of Ca2+ channels in rat phaeochromocytoma cell lines. J Physiol. 1990 Jul;426:95–116. doi: 10.1113/jphysiol.1990.sp018128. [DOI] [PMC free article] [PubMed] [Google Scholar]


