Abstract
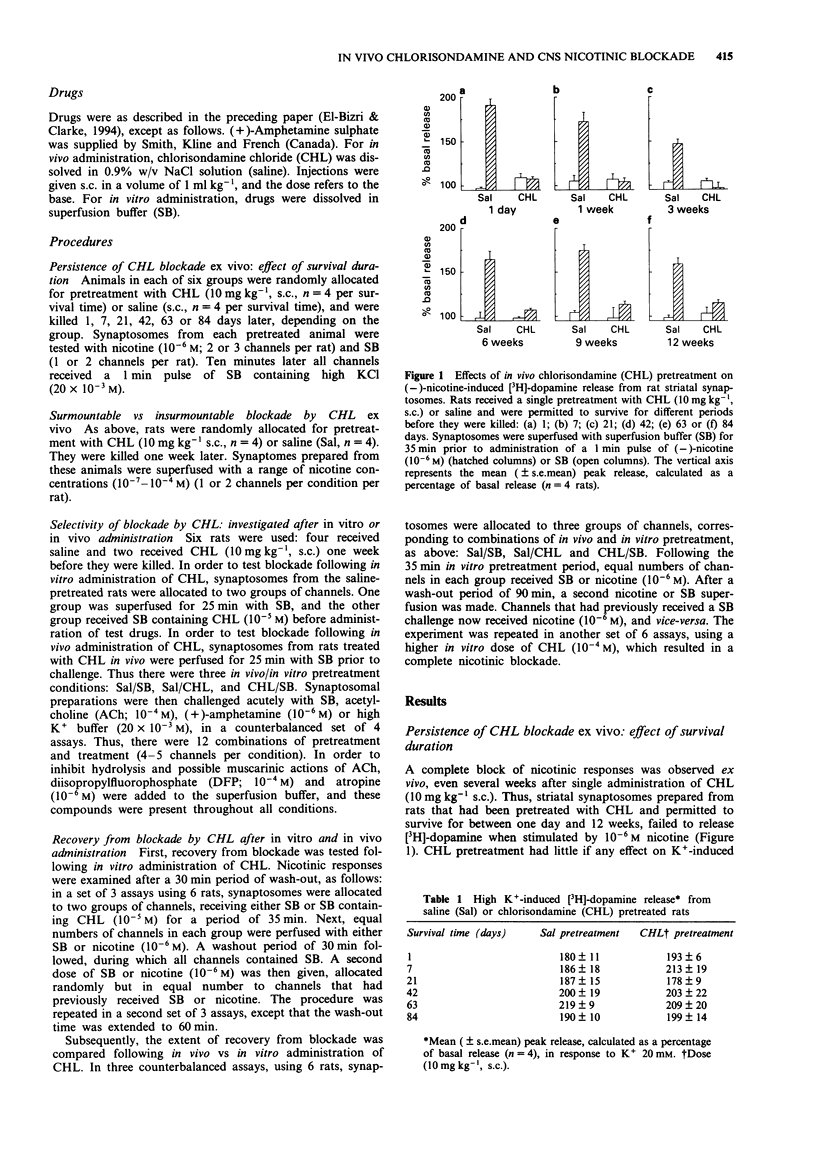
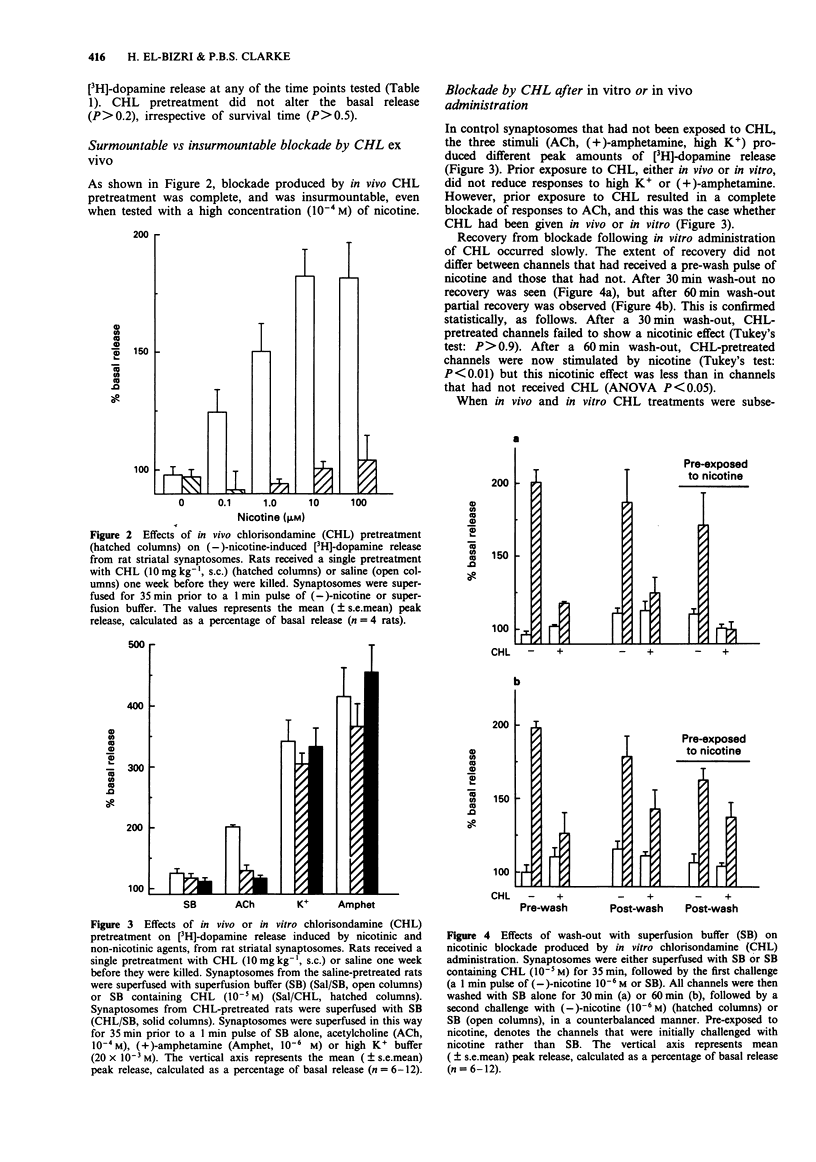
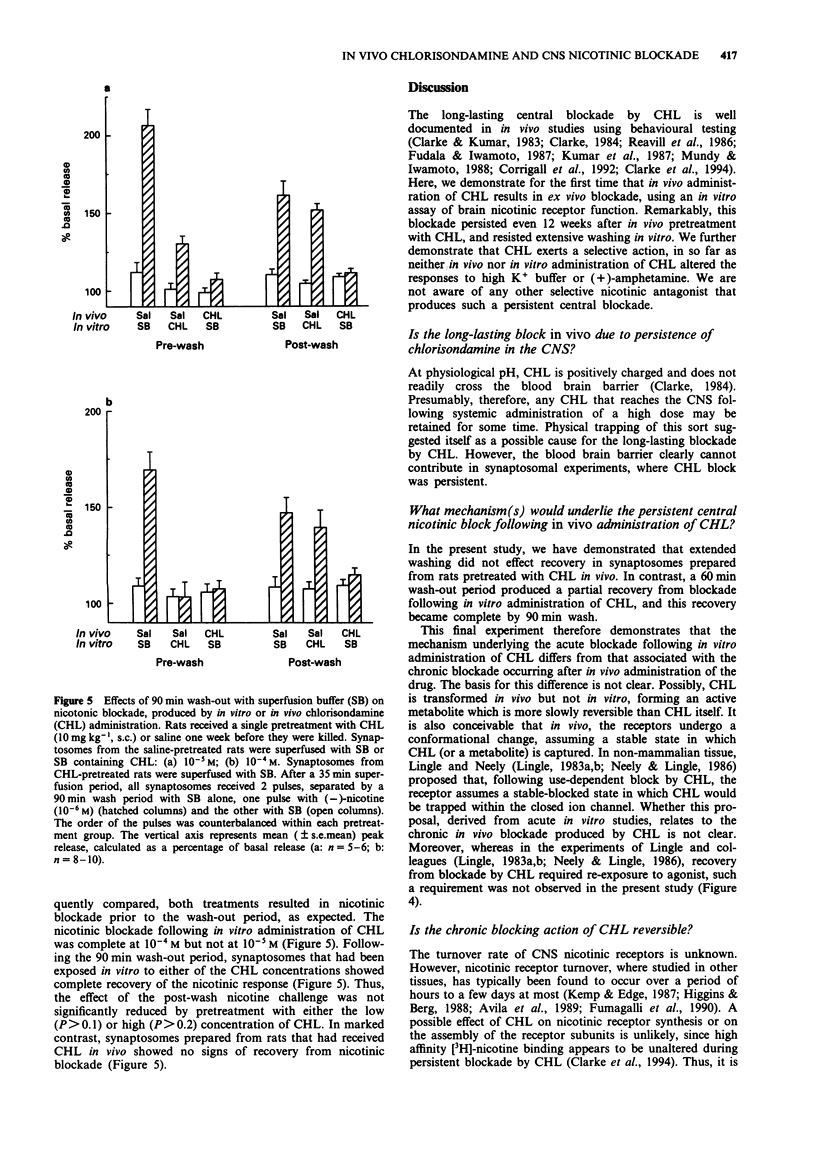
1. The chronic nicotinic blockade produced following in vivo administration of chlorisondamine was investigated in vitro. Nicotine-induced [3H]-dopamine release from striatal synaptosomes was used as a measure of central nicotinic receptor function. 2. In synaptosomal preparations from rats pretreated with a single administration of chlorisondamine (10 mg kg-1, s.c.), 1, 7, 21, 42, 63 or 84 days before they were killed, responses to (-)-nicotine (10(-6) M) were blocked. 3. In vivo administration of chlorisondamine (10 mg kg-1, s.c.), 7 days before rats were killed, produced a nicotinic blockade in vitro that was insurmountable even with a high concentration of (-)-nicotine (10(-4) M). 4. Both in vitro and in vivo administration of chlorisondamine blocked nicotinic responses to acetylcholine (10(-4) M). In contrast, neither in vitro nor in vivo administration of chlorisondamine reduced [3H]-dopamine release induced by high K+ (20 x 10(-3) M) or (+)-amphetamine (10(-6) M). 5. Nicotinic blockade resulting from in vitro administration of chlorisondamine (10(-5) M) recovered partially after 60 min wash-out, and completely by 90 min. In contrast, no recovery was seen in synaptosomes prepared from rats pretreated with chlorisondamine (10 mg kg-1, s.c.) in vivo. 6. Thus, in vivo treatment with chlorisondamine results in a quasi-irreversible, insurmountable block of CNS nicotinic receptors. The persistence of this block ex vivo indicates that physical trapping by the blood brain barrier is not solely responsible for the persistent blockade seen in vivo.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila O. L., Drachman D. B., Pestronk A. Neurotransmission regulates stability of acetylcholine receptors at the neuromuscular junction. J Neurosci. 1989 Aug;9(8):2902–2906. doi: 10.1523/JNEUROSCI.09-08-02902.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. B., Chaudieu I., el-Bizri H., Boksa P., Quik M., Esplin B. A., Capek R. The pharmacology of the nicotinic antagonist, chlorisondamine, investigated in rat brain and autonomic ganglion. Br J Pharmacol. 1994 Feb;111(2):397–405. doi: 10.1111/j.1476-5381.1994.tb14748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. B. Chronic central nicotinic blockade after a single administration of the bisquaternary ganglion-blocking drug chlorisondamine. Br J Pharmacol. 1984 Oct;83(2):527–535. doi: 10.1111/j.1476-5381.1984.tb16517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. B., Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol. 1983 Nov;80(3):587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall W. A., Franklin K. B., Coen K. M., Clarke P. B. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107(2-3):285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Fudala P. J., Iwamoto E. T. Conditioned aversion after delay place conditioning with nicotine. Psychopharmacology (Berl) 1987;92(3):376–381. doi: 10.1007/BF00210847. [DOI] [PubMed] [Google Scholar]
- Fumagalli G., Balbi S., Cangiano A., Lømo T. Regulation of turnover and number of acetylcholine receptors at neuromuscular junctions. Neuron. 1990 Apr;4(4):563–569. doi: 10.1016/0896-6273(90)90114-u. [DOI] [PubMed] [Google Scholar]
- Giorguieff-Chesselet M. F., Kemel M. L., Wandscheer D., Glowinski J. Regulation of dopamine release by presynaptic nicotinic receptors in rat striatal slices: effect of nicotine in a low concentration. Life Sci. 1979 Oct 1;25(14):1257–1262. doi: 10.1016/0024-3205(79)90469-7. [DOI] [PubMed] [Google Scholar]
- Giorguieff M. F., Le Floc'h M. L., Glowinski J., Besson M. J. Involvement of cholinergic presynaptic receptors of nicotinic and muscarinic types in the control of the spontaneous release of dopamine from striatal dopaminergic terminals in the rat. J Pharmacol Exp Ther. 1977 Mar;200(3):535–544. [PubMed] [Google Scholar]
- Grady S., Marks M. J., Wonnacott S., Collins A. C. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992 Sep;59(3):848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Higgins L. S., Berg D. K. Metabolic stability and antigenic modulation of nicotinic acetylcholine receptors on bovine adrenal chromaffin cells. J Cell Biol. 1988 Sep;107(3):1147–1156. doi: 10.1083/jcb.107.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp G., Edge M. Cholinergic function and alpha-bungarotoxin binding in PC12 cells. Mol Pharmacol. 1987 Sep;32(3):356–363. [PubMed] [Google Scholar]
- Kumar R., Reavill C., Stolerman I. P. Nicotine cue in rats: effects of central administration of ganglion-blocking drugs. Br J Pharmacol. 1987 Jan;90(1):239–246. doi: 10.1111/j.1476-5381.1987.tb16845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C. Blockade of cholinergic channels by chlorisondamine on a crustacean muscle. J Physiol. 1983 Jun;339:395–417. doi: 10.1113/jphysiol.1983.sp014723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C. Different types of blockade of crustacean acetylcholine-induced currents. J Physiol. 1983 Jun;339:419–437. doi: 10.1113/jphysiol.1983.sp014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy W. R., Iwamoto E. T. Actions of nicotine on the acquisition of an autoshaped lever-touch response in rats. Psychopharmacology (Berl) 1988;94(2):267–274. doi: 10.1007/BF00176858. [DOI] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Trapping of an open-channel blocker at the frog neuromuscular acetylcholine channel. Biophys J. 1986 Nov;50(5):981–986. doi: 10.1016/S0006-3495(86)83538-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER A. J., TRAPOLD J. H., SCHNEIDER J. A., MAXWELL R. A., EARL A. E. Ganglionic blockade by a new bisquaternary series, including chlorisondamine dimethochloride. J Pharmacol Exp Ther. 1955 Oct;115(2):172–184. [PubMed] [Google Scholar]
- Rapier C., Lunt G. G., Wonnacott S. Stereoselective nicotine-induced release of dopamine from striatal synaptosomes: concentration dependence and repetitive stimulation. J Neurochem. 1988 Apr;50(4):1123–1130. doi: 10.1111/j.1471-4159.1988.tb10582.x. [DOI] [PubMed] [Google Scholar]
- Reavill C., Stolerman I. P., Kumar R., Garcha H. S. Chlorisondamine blocks acquisition of the conditioned taste aversion produced by (-)-nicotine. Neuropharmacology. 1986 Sep;25(9):1067–1069. doi: 10.1016/0028-3908(86)90204-2. [DOI] [PubMed] [Google Scholar]
- el-Bizri H., Clarke P. B. Blockade of nicotinic receptor-mediated release of dopamine from striatal synaptosomes by chlorisondamine and other nicotinic antagonists administered in vitro. Br J Pharmacol. 1994 Feb;111(2):406–413. doi: 10.1111/j.1476-5381.1994.tb14749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


