Abstract
1. The sensitivity of long-term potentiation (LTP) to nitric oxide synthase (NOS) inhibition was determined for two synaptic input systems onto CA3 pyramidal neurones the LTP of which display differential sensitivity to N-methyl-D-aspartate (NMDA) receptor antagonists: the fimbrial input which activates the associational-commissural synapses on the distal apical dendrites and the mossy fibre input which synapses on the proximal apical dendrites of CA3 pyramidal neurones. 2. Following high-frequency stimulation (HFS) of the fimbrial input, average e.p.s.p. amplitude increased by 92.4 +/- 22.0% (mean +/- s.e.mean; n = 6 cells) when compared to the pre-HFS average. In the presence of 100 microM N omega-nitro-L-arginine methyl ester (L-NAME), the enhancement was reduced significantly to 32.2 +/- 11.6% (n = 5 cells; P < 0.05). In the presence of 300 microM L-NAME, the inhibition was more complete, with post-HFS e.p.s.p. amplitude increasing an average 6.2 +/- 9.3% (n = 7 cells, P < 0.05). 3. Following high frequency stimulation of the mossy fibre input, average e.p.s.p. amplitude increased by 57.9 +/- 13.0% (n = 6 cells) when compared to the pre-HFS average. The presence of 100 microM L-NAME had no significant effect on the enhancement, averaging 63.6 +/- 5.9% (n = 4 cells; P > 0.05). Similarly, increasing the concentration of L-NAME to 300 microM had no significant effect on the potentiation, with the post-HFS amplitude increasing by an average 55.6 +/- 9.5% (n = 5 cells, P > 0.05).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
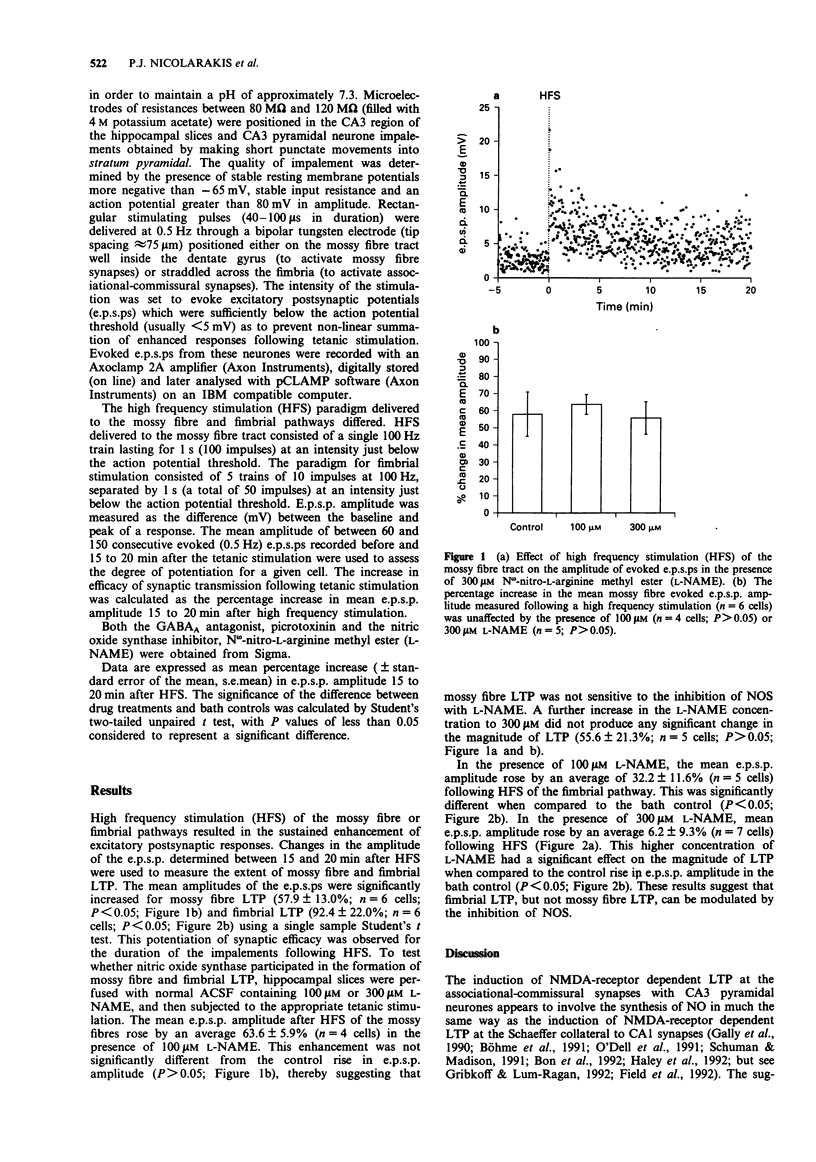
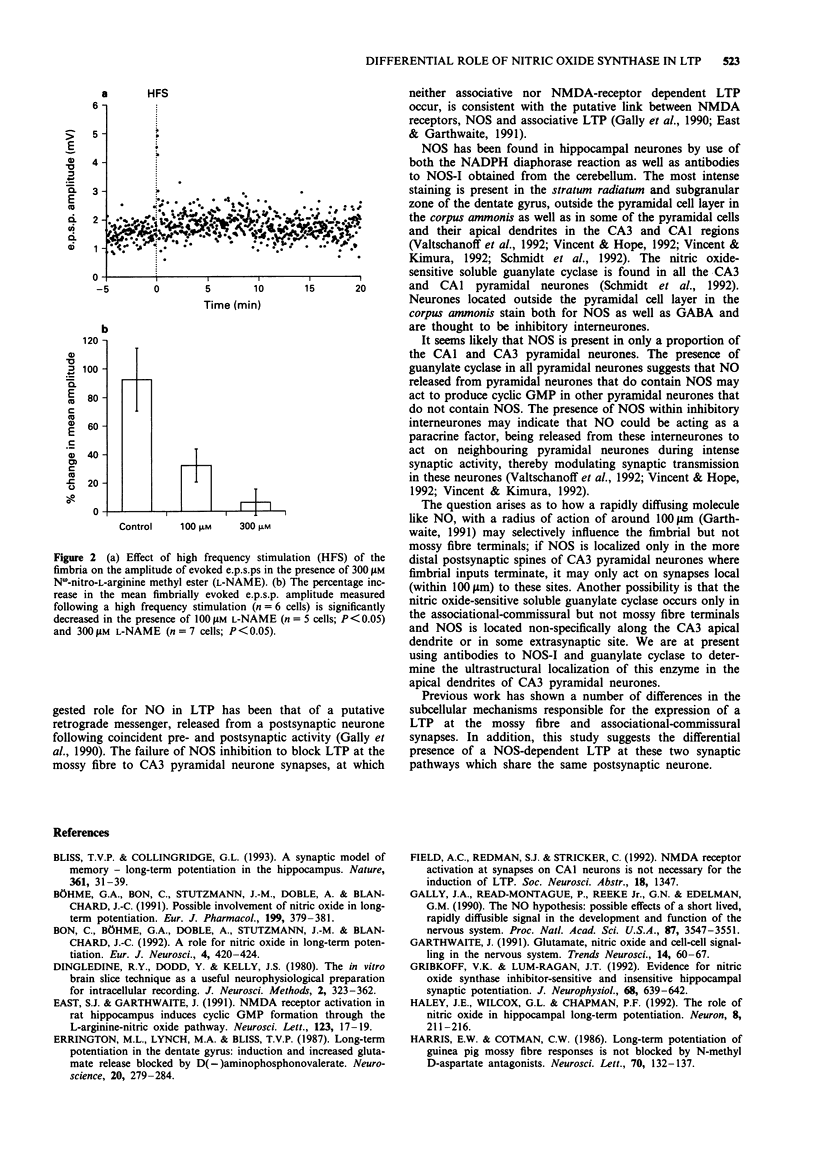

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bon Christelle, Böhme Georg Andrees, Doble Adam, Stutzmann Jean-Marie, Blanchard Jean-Charles. A Role for Nitric Oxide in Long-term Potentiation. Eur J Neurosci. 1992;4(5):420–424. doi: 10.1111/j.1460-9568.1992.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Böhme G. A., Bon C., Stutzmann J. M., Doble A., Blanchard J. C. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol. 1991 Jul 9;199(3):379–381. doi: 10.1016/0014-2999(91)90505-k. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Dodd J., Kelly J. S. The in vitro brain slice as a useful neurophysiological preparation for intracellular recording. J Neurosci Methods. 1980 Aug;2(4):323–362. doi: 10.1016/0165-0270(80)90002-3. [DOI] [PubMed] [Google Scholar]
- East S. J., Garthwaite J. NMDA receptor activation in rat hippocampus induces cyclic GMP formation through the L-arginine-nitric oxide pathway. Neurosci Lett. 1991 Feb 11;123(1):17–19. doi: 10.1016/0304-3940(91)90147-l. [DOI] [PubMed] [Google Scholar]
- Errington M. L., Lynch M. A., Bliss T. V. Long-term potentiation in the dentate gyrus: induction and increased glutamate release are blocked by D(-)aminophosphonovalerate. Neuroscience. 1987 Jan;20(1):279–284. doi: 10.1016/0306-4522(87)90019-4. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Montague P. R., Reeke G. N., Jr, Edelman G. M. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990 May;87(9):3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gribkoff V. K., Lum-Ragan J. T. Evidence for nitric oxide synthase inhibitor-sensitive and insensitive hippocampal synaptic potentiation. J Neurophysiol. 1992 Aug;68(2):639–642. doi: 10.1152/jn.1992.68.2.639. [DOI] [PubMed] [Google Scholar]
- Haley J. E., Wilcox G. L., Chapman P. F. The role of nitric oxide in hippocampal long-term potentiation. Neuron. 1992 Feb;8(2):211–216. doi: 10.1016/0896-6273(92)90288-o. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Cotman C. W. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986 Sep 25;70(1):132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Jaffe D., Johnston D. Induction of long-term potentiation at hippocampal mossy-fiber synapses follows a Hebbian rule. J Neurophysiol. 1990 Sep;64(3):948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- Johnston D., Williams S., Jaffe D., Gray R. NMDA-receptor-independent long-term potentiation. Annu Rev Physiol. 1992;54:489–505. doi: 10.1146/annurev.ph.54.030192.002421. [DOI] [PubMed] [Google Scholar]
- Katsuki H., Kaneko S., Satoh M. Involvement of postsynaptic G proteins in hippocampal long-term potentiation. Brain Res. 1992 May 22;581(1):108–114. doi: 10.1016/0006-8993(92)90349-e. [DOI] [PubMed] [Google Scholar]
- Katsuki H., Kaneko S., Tajima A., Satoh M. Separate mechanisms of long-term potentiation in two input systems to CA3 pyramidal neurons of rat hippocampal slices as revealed by the whole-cell patch-clamp technique. Neurosci Res. 1991 Nov;12(3):393–402. doi: 10.1016/0168-0102(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Gagne G. D., Nakane M., Pollock J. S., Miller M. F., Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992 Oct;40(10):1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hope B. T. Neurons that say NO. Trends Neurosci. 1992 Mar;15(3):108–113. doi: 10.1016/0166-2236(92)90021-y. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46(4):755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Zalutsky R. A., Nicoll R. A. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990 Jun 29;248(4963):1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zalutsky R. A., Nicoll R. A. Mossy fiber long-term potentiation shows specificity but no apparent cooperativity. Neurosci Lett. 1992 Apr 13;138(1):193–197. doi: 10.1016/0304-3940(92)90503-y. [DOI] [PubMed] [Google Scholar]


