Abstract
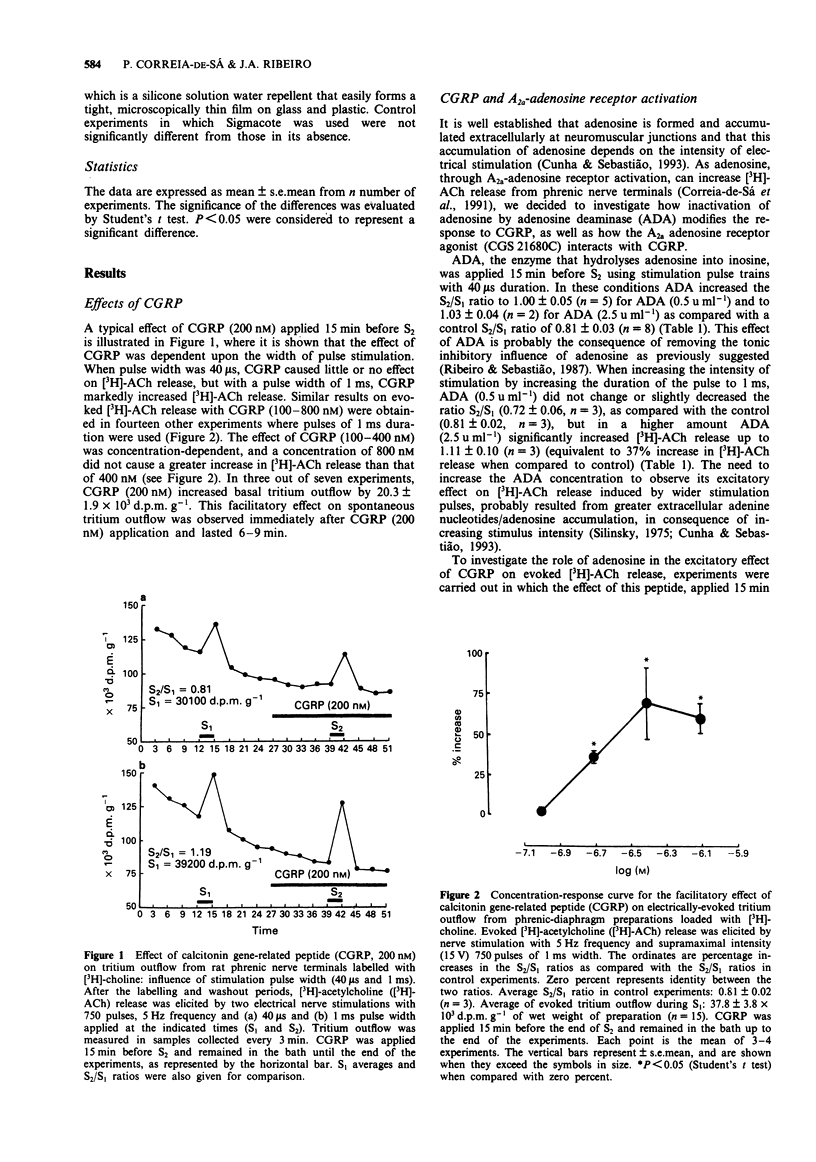
1. The effect of calcitonin gene-related peptide (CGRP) on [3H]-acetylcholine ([3H]-ACh) release from motor nerve endings and its interaction with presynaptic facilitatory A2a-adenosine and nicotinic acetylcholine receptors was studied on rat phrenic nerve-hemidiaphragm preparations loaded with [3H]-choline. 2. CGRP (100-400 nM) increased electrically evoked [3H]-ACh release from phrenic nerve endings in a concentration-dependent manner. 3. The magnitude of CGRP excitation increased with the increase of the stimulation pulse duration from 40 microseconds to 1 ms, keeping the frequency, the amplitude and the train length constants. With 1 ms pulses, the evoked [3H]-ACh release was more intense than with 40 microseconds pulse duration. 4. Both the nicotinic acetylcholine receptor agonist, 1,1-dimethyl-4-phenylpiperazinium, and the A2a adenosine receptor agonist, CGS 21680C, increased evoked [3H]-ACh release, but only CGS 21680C potentiated the facilitatory effect of CGRP. This potentiation was prevented by the A2a adenosine receptor antagonist, PD 115,199. 5. Adenosine deaminase prevented the excitatory effect of CGRP (400 nM) on [3H]-ACh release. This effect was reversed by the non-hydrolysable A2a-adenosine receptor agonist, CGS 21680C. 6. The nicotinic antagonist, tubocurarine, did not significantly change, whereas the A2-adenosine receptor antagonist, PD 115,199, blocked the CGRP facilitation. The A1-adenosine receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine, potentiated the CGRP excitatory effect. 7. The results suggest that the facilitatory effect of CGRP on evoked [3H]-ACh release from rat phrenic motor nerve endings depends on the presence of endogenous adenosine which tonically activates A2a-adenosine receptors.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

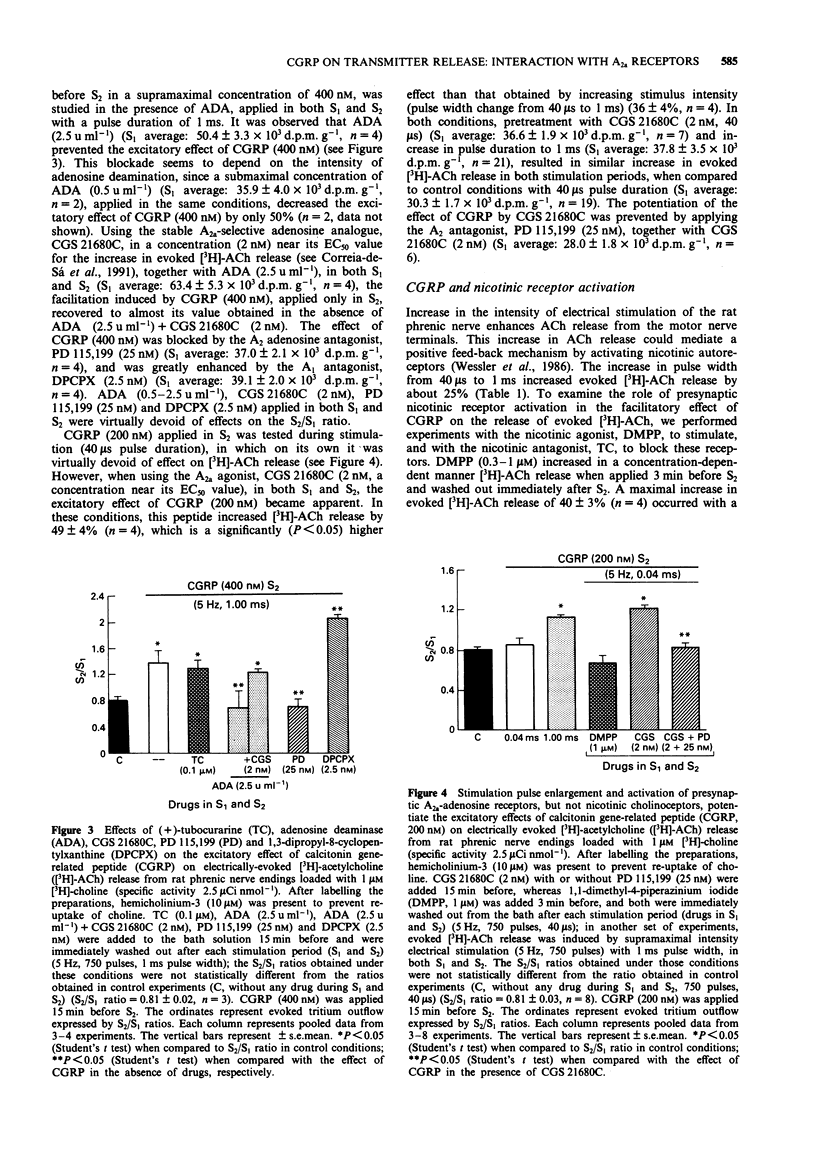



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alousi A. A., Jasper J. R., Insel P. A., Motulsky H. J. Stoichiometry of receptor-Gs-adenylate cyclase interactions. FASEB J. 1991 Jun;5(9):2300–2303. doi: 10.1096/fasebj.5.9.1650314. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Battaglia G., Norman A. B., Hess E. J., Creese I. Forskolin potentiates the stimulation of rat striatal adenylate cyclase mediated by D-1 dopamine receptors, guanine nucleotides, and sodium fluoride. J Neurochem. 1986 Apr;46(4):1180–1185. doi: 10.1111/j.1471-4159.1986.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P., Ribeiro J. A. Facilitation of [3H]-ACh release by forskolin depends on A2-adenosine receptor activation. Neurosci Lett. 1993 Mar 5;151(1):21–24. doi: 10.1016/0304-3940(93)90035-j. [DOI] [PubMed] [Google Scholar]
- Correia-de-Sá P., Sebastião A. M., Ribeiro J. A. Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve ending of the rat. Br J Pharmacol. 1991 Jun;103(2):1614–1620. doi: 10.1111/j.1476-5381.1991.tb09836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha R. A., Sebastião A. M. Adenosine and adenine nucleotides are independently released from both the nerve terminals and the muscle fibres upon electrical stimulation of the innervated skeletal muscle of the frog. Pflugers Arch. 1993 Sep;424(5-6):503–510. doi: 10.1007/BF00374914. [DOI] [PubMed] [Google Scholar]
- Jinnai K., Chihara K., Kanda F., Tada K., Fujita T. Calcitonin gene-related peptide enhances spontaneous acetylcholine release from the rat motor nerve terminal. Neurosci Lett. 1989 Aug 14;103(1):64–68. doi: 10.1016/0304-3940(89)90486-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Hashimoto K., Uchida S., Sakuma J., Takami K., Tohyama M., Izumi F., Yoshida H. Calcitonin gene related peptide stimulates adenylate cyclase activity in rat striated muscle. Experientia. 1987 Mar 15;43(3):314–316. doi: 10.1007/BF01945565. [DOI] [PubMed] [Google Scholar]
- Mulholland M. W., Jaffer S. Stimulation of acetylcholine release in myenteric plexus by calcitonin gene-related peptide. Am J Physiol. 1990 Dec;259(6 Pt 1):G934–G939. doi: 10.1152/ajpgi.1990.259.6.G934. [DOI] [PubMed] [Google Scholar]
- Ohhashi T., Jacobowitz D. M. Effects of calcitonin gene-related peptide on neuromuscular transmission in the isolated rat diaphragm. Peptides. 1988 May-Jun;9(3):613–617. doi: 10.1016/0196-9781(88)90172-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol. 1987 Mar;384:571–585. doi: 10.1113/jphysiol.1987.sp016470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O. Sources of adenosine released during neuromuscular transmission in the rat. J Physiol. 1991 Jan;432:343–354. doi: 10.1113/jphysiol.1991.sp018388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H., Kojima M., Nagata M., Kuromi H. On the site of action of hemicholinium-3 at the rat phrenic nerve-diaphragm preparation with special reference to its multiple presynaptic actions. Neuropharmacology. 1970 Jul;9(4):359–367. doi: 10.1016/0028-3908(70)90033-x. [DOI] [PubMed] [Google Scholar]
- Takami K., Hashimoto K., Uchida S., Tohyama M., Yoshida H. Effect of calcitonin gene-related peptide on the cyclic AMP level of isolated mouse diaphragm. Jpn J Pharmacol. 1986 Nov;42(3):345–350. doi: 10.1254/jjp.42.345. [DOI] [PubMed] [Google Scholar]
- Takami K., Kawai Y., Uchida S., Tohyama M., Shiotani Y., Yoshida H., Emson P. C., Girgis S., Hillyard C. J., MacIntyre I. Effect of calcitonin gene-related peptide on contraction of striated muscle in the mouse. Neurosci Lett. 1985 Sep 30;60(2):227–230. doi: 10.1016/0304-3940(85)90248-4. [DOI] [PubMed] [Google Scholar]
- Wessler I. Control of transmitter release from the motor nerve by presynaptic nicotinic and muscarinic autoreceptors. Trends Pharmacol Sci. 1989 Mar;10(3):110–114. doi: 10.1016/0165-6147(89)90208-3. [DOI] [PubMed] [Google Scholar]
- Wessler I., Halank M., Rasbach J., Kilbinger H. Presynaptic nicotine receptors mediating a positive feed-back on transmitter release from the rat phrenic nerve. Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):365–372. doi: 10.1007/BF00569371. [DOI] [PubMed] [Google Scholar]
- Wessler I., Kilbinger H. Release of [3H]acetylcholine from a modified rat phrenic nerve-hemidiaphragm preparation. Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):357–364. doi: 10.1007/BF00569370. [DOI] [PubMed] [Google Scholar]
- Wiley J. W., Gross R. A., MacDonald R. L. The peptide CGRP increases a high-threshold Ca2+ current in rat nodose neurones via a pertussis toxin-sensitive pathway. J Physiol. 1992 Sep;455:367–381. doi: 10.1113/jphysiol.1992.sp019306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F. The effects of dibutyryl cyclic adenosine 3',5'-monophosphate, theophylline and aminophylline on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1974 Feb;188(2):447–452. [PubMed] [Google Scholar]


