Abstract
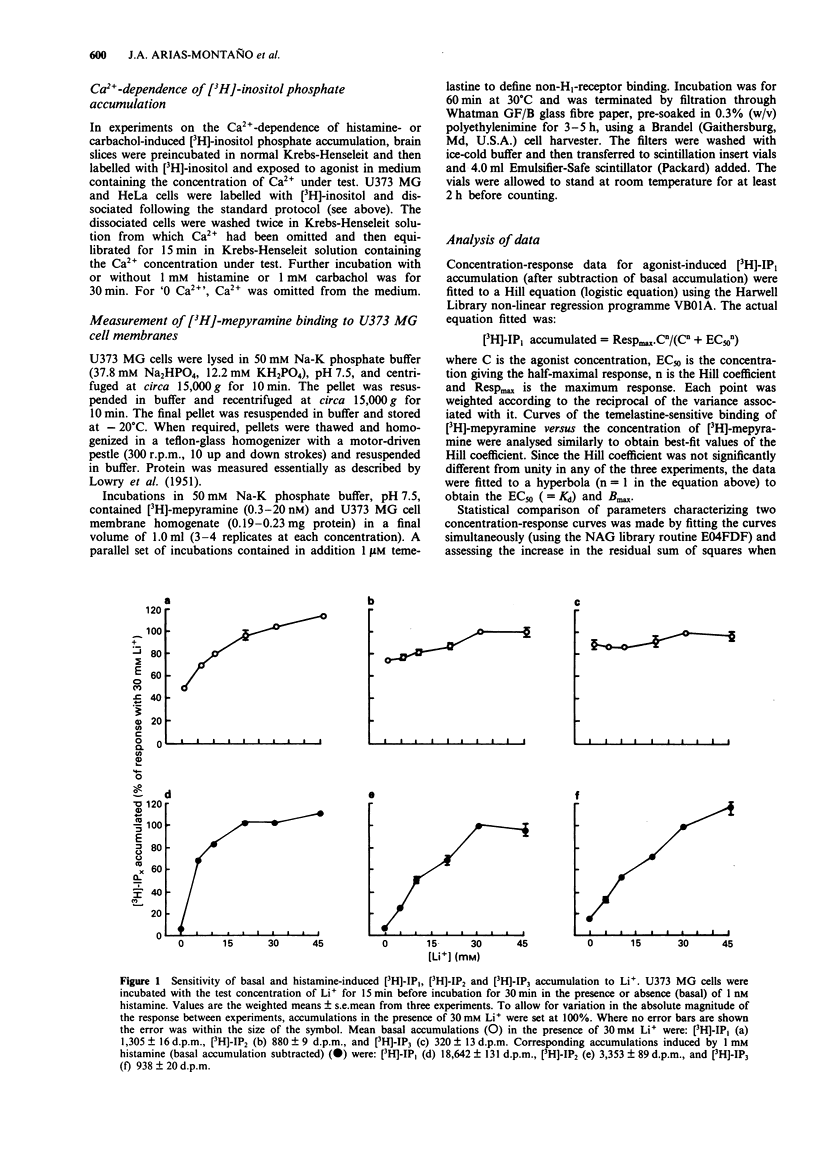
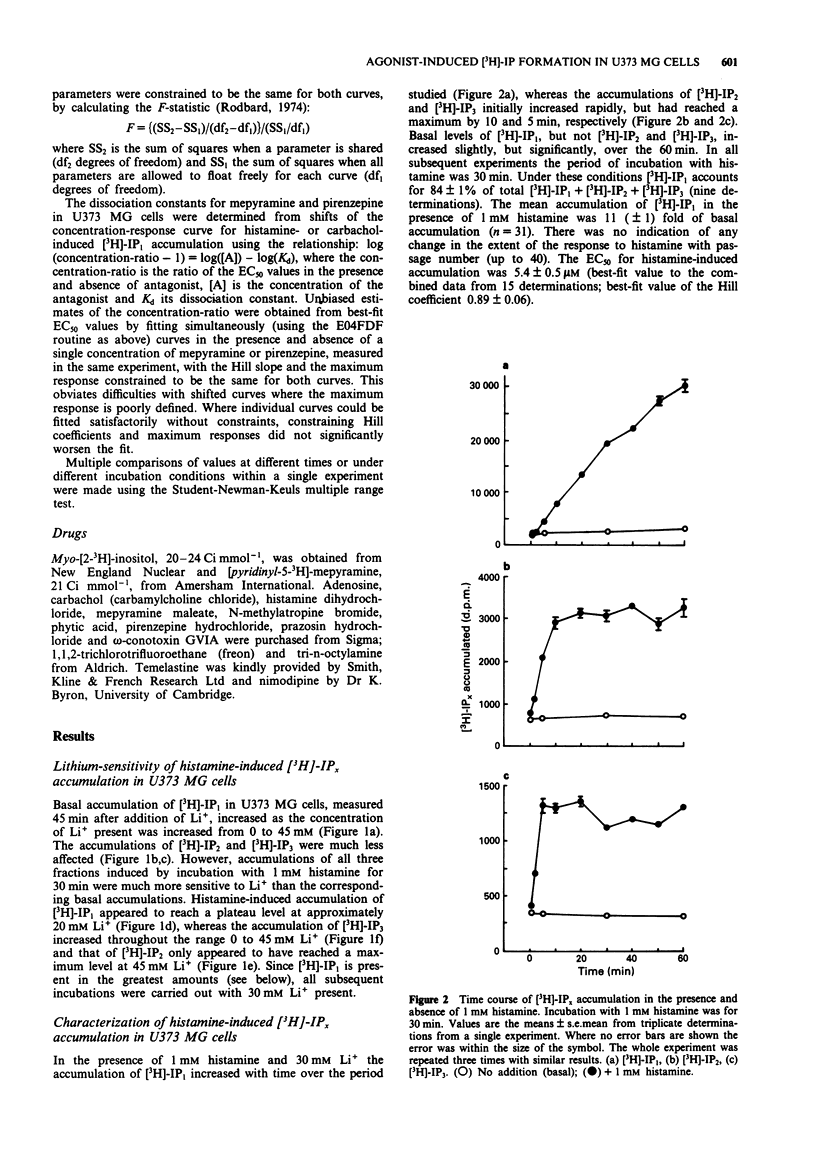
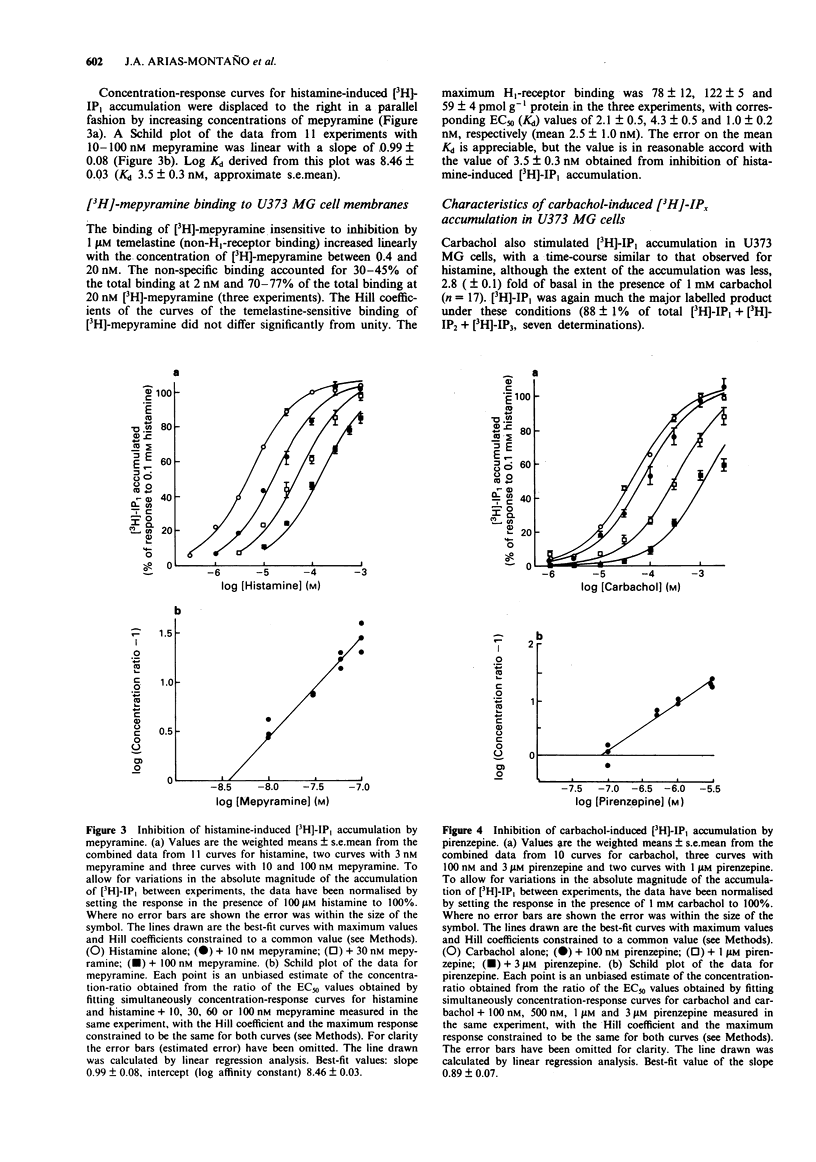
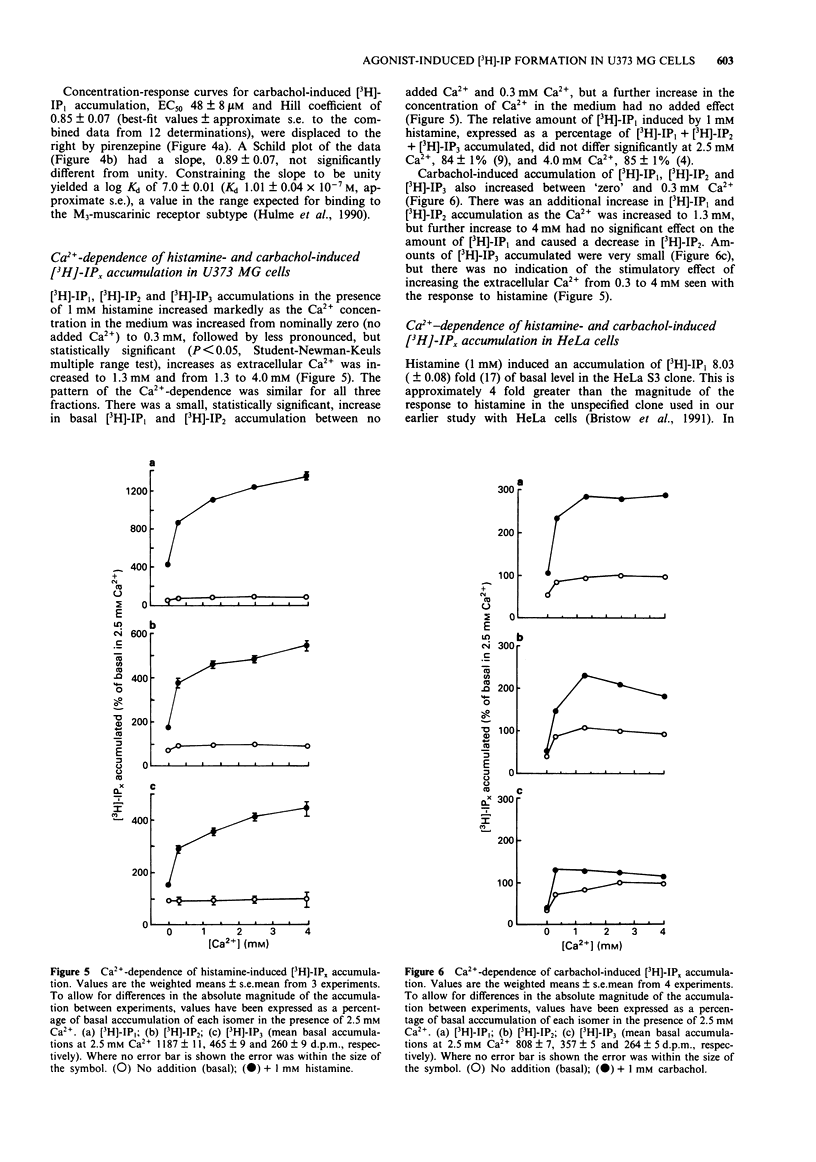
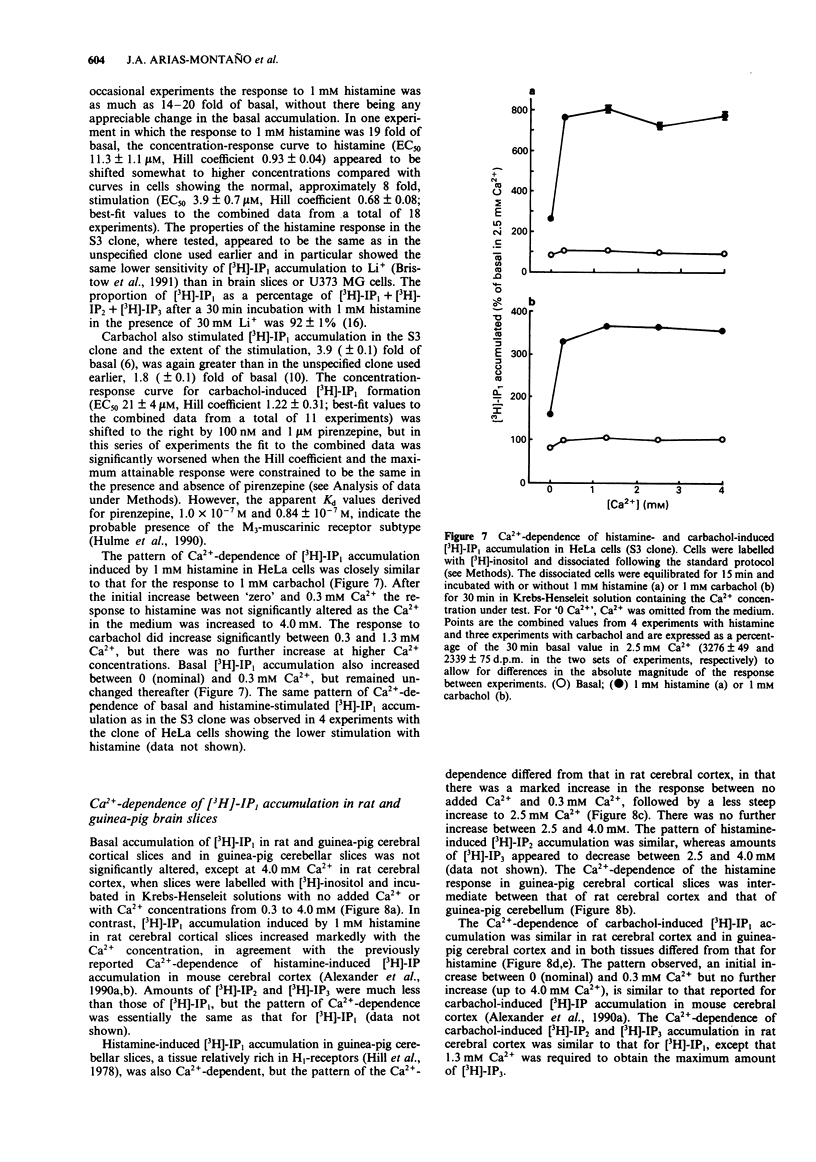
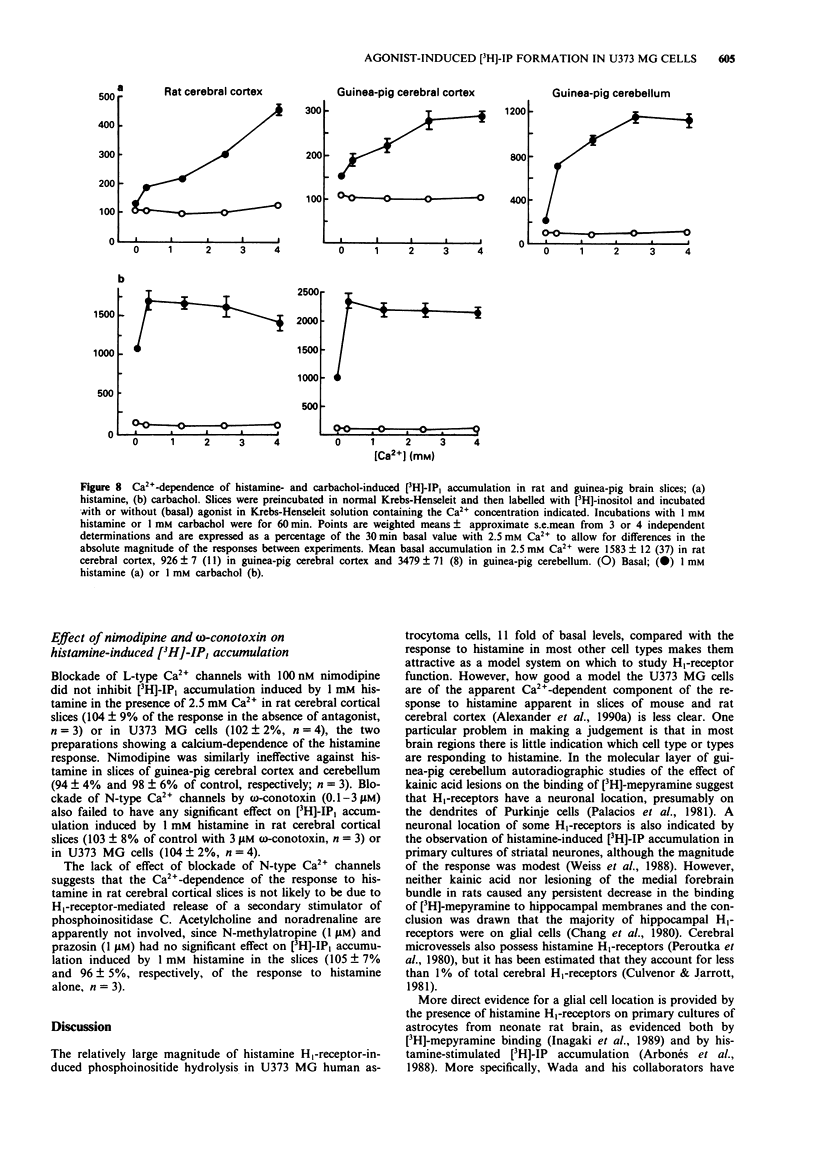
1. Histamine (1 mM) induced an accumulation of inositol monophosphate ([3H]-IP1) in the U373 MG human astrocytoma cell line which increased with time in the presence of 30 mM Li+. After a 30 min incubation period with 1 mM histamine [3H]-IP1 was the major product detected (84 +/- 1% of total [3H]-IPx) and was present at a level 11 (+/- 1) fold of basal accumulation. 2. Concentration-response curves for histamine-induced [3H]-IP1 accumulation in U373 MG cells (EC50 5.4 +/- 0.5 microM) were shifted to the right in a parallel fashion by mepyramine (slope of a Schild plot 0.99 +/- 0.08), yielding a Kd for mepyramine of 3.5 +/- 0.3 nM, consistent with the involvement of histamine H1-receptors. 3. The temelastine-sensitive binding of [3H]-mepyramine to a membrane fraction from U373 MG cells was hyperbolic and had a mean Kd of 2.5 +/- 1.0 nM. The maximum amount of temelastine-sensitive binding was 86 +/- 19 pmol g-1 membrane protein. 4. Carbachol also induced [3H]-IP1 accumulation in U373 MG cells, 2.8 (+/- 0.1) fold of basal with 1 mM carbachol, with an EC50 of 48 +/- 8 microM. Pirenzepine shifted carbachol concentration-response curves to the right (slope of Schild plot 0.89 +/- 0.07) giving a Kd for pirenzepine of 0.10 +/- 0.01 microM, suggesting that phosphoinositide hydrolysis in U373 MG cells is mediated by the M3-, rather than the M1-, muscarinic receptor subtype. 5. [3H]-IP1 accumulation induced by both 1 mM histamine and by 1 mM carbachol increased when the Ca2+ concentration of the medium was increased from 'zero' (no added Ca2+) to 0.3 mM. Histamine-stimulated [3H]-IP1 accumulation was further increased, although not so markedly, as the Ca2+ was raised to 4 mM. The same pattern was apparent with histamine-induced accumulations of [3H]-IP2 and [3H]-IP3. In contrast, [3H]-IPx accumulation in response to carbachol increased between 0.3 and 1.3 mM, but thereafter remained unchanged ([3H]-IP1) or declined ([3H]-IP2 and [3H]-IP3). 6. In HeLa cells, [3H]-IP1 accumulations induced by 1 mM histamine and 1 mM carbachol showed the same pattern of Ca2+ dependence and were independent of extracellular Ca2+ above 0.3 mM (histamine) or 1.3 mM (carbachol). The response to carbachol appeared to be mediated by an M3-muscarinic receptor (apparent Kd for pirenzepine 0.09 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. P., Hill S. J., Kendall D. A. Differential effects of elevated calcium ion concentrations on inositol phospholipid responses in mouse and rat cerebral cortical slices. Biochem Pharmacol. 1990 Oct 15;40(8):1793–1799. doi: 10.1016/0006-2952(90)90358-r. [DOI] [PubMed] [Google Scholar]
- Alexander S. P., Hill S. J., Kendall D. A. Is the adenosine receptor modulation of histamine-induced accumulation of inositol phosphates in cerebral cortical slices mediated by effects on calcium ion fluxes? J Neurochem. 1990 Oct;55(4):1138–1141. doi: 10.1111/j.1471-4159.1990.tb03116.x. [DOI] [PubMed] [Google Scholar]
- Arbonés L., Picatoste F., García A. Histamine H1-receptors mediate phosphoinositide hydrolysis in astrocyte-enriched primary cultures. Brain Res. 1988 May 31;450(1-2):144–152. doi: 10.1016/0006-8993(88)91554-5. [DOI] [PubMed] [Google Scholar]
- Arbonés L., Picatoste F., García A. Histamine stimulates glycogen breakdown and increases 45Ca2+ permeability in rat astrocytes in primary culture. Mol Pharmacol. 1990 Jun;37(6):921–927. [PubMed] [Google Scholar]
- Arias-Montaño J. A., Young J. M. Characteristics of histamine H1 receptors on HeLa cells. Eur J Pharmacol. 1993 May 15;245(3):291–295. doi: 10.1016/0922-4106(93)90110-u. [DOI] [PubMed] [Google Scholar]
- Arias-Montaño J. A., Young J. M. Locus of action of Ni2+ on histamine-induced inositol phosphate formation in brain slices and in HeLa cells. Eur J Pharmacol. 1993 May 15;245(3):221–228. doi: 10.1016/0922-4106(93)90100-n. [DOI] [PubMed] [Google Scholar]
- Baird J. G., Chilvers E. R., Kennedy E. D., Nahorski S. R. Changes in extracellular calcium within the physiological range influence receptor-mediated inositol phosphate responses in brain and tracheal smooth muscle slices. Naunyn Schmiedebergs Arch Pharmacol. 1989 Mar;339(3):247–251. doi: 10.1007/BF00173572. [DOI] [PubMed] [Google Scholar]
- Baird J. G., Nahorski S. R. Increased intracellular calcium stimulates 3H-inositol polyphosphate accumulation in rat cerebral cortical slices. J Neurochem. 1990 Feb;54(2):555–561. doi: 10.1111/j.1471-4159.1990.tb01907.x. [DOI] [PubMed] [Google Scholar]
- Bristow D. R., Arias-Montaño J. A., Young J. M. Histamine-induced inositol phosphate accumulation in HeLa cells: lithium sensitivity. Br J Pharmacol. 1991 Nov;104(3):677–684. doi: 10.1111/j.1476-5381.1991.tb12488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Tran V. T., Snyder S. H. Neurotransmitter receptor localizations: brain lesion induced alterations in benzodiazepine, GABA, beta-adrenergic and histamine H1-receptor binding. Brain Res. 1980 May 19;190(1):95–110. doi: 10.1016/0006-8993(80)91162-2. [DOI] [PubMed] [Google Scholar]
- Daum P. R., Downes C. P., Young J. M. Histamine-induced inositol phospholipid breakdown mirrors H1-receptor density in brain. Eur J Pharmacol. 1983 Mar 4;87(4):497–498. doi: 10.1016/0014-2999(83)90092-4. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Intracellular Ca2+ activates phospholipase C. Trends Neurosci. 1988 Dec;11(12):517–520. doi: 10.1016/0166-2236(88)90174-9. [DOI] [PubMed] [Google Scholar]
- Forray C., el-Fakahany E. E. On the involvement of multiple muscarinic receptor subtypes in the activation of phosphoinositide metabolism in rat cerebral cortex. Mol Pharmacol. 1990 Jun;37(6):893–902. [PubMed] [Google Scholar]
- Goh Y., Kurosawa A. Characterization and Ca2+ requirement of histamine-induced catecholamine secretion in cultured bovine chromaffin cells. J Neurochem. 1991 Oct;57(4):1249–1257. doi: 10.1111/j.1471-4159.1991.tb08286.x. [DOI] [PubMed] [Google Scholar]
- Hill S. J. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990 Mar;42(1):45–83. [PubMed] [Google Scholar]
- Hill S. J., Emson P. C., Young J. M. The binding of [3H]mepyramine to histamine H1 receptors in guinea-pig brain. J Neurochem. 1978 Oct;31(4):997–1004. doi: 10.1111/j.1471-4159.1978.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Fukui H., Ito S., Yamatodani A., Wada H. Single type-2 astrocytes show multiple independent sites of Ca2+ signaling in response to histamine. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4215–4219. doi: 10.1073/pnas.88.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Fukui H., Taguchi Y., Wang N. P., Yamatodani A., Wada H. Characterization of histamine H1-receptors on astrocytes in primary culture: [3H]mepyramine binding studies. Eur J Pharmacol. 1989 Nov 28;173(1):43–51. doi: 10.1016/0014-2999(89)90007-1. [DOI] [PubMed] [Google Scholar]
- Johnson C. L., Johnson C. G. Characterization of receptors for substance P in human astrocytoma cells: radioligand binding and inositol phosphate formation. J Neurochem. 1992 Feb;58(2):471–477. doi: 10.1111/j.1471-4159.1992.tb09745.x. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Firth J. L. Inositol phospholipid hydrolysis in human brain; adenosine inhibition of the response to histamine. Br J Pharmacol. 1990 May;100(1):37–40. doi: 10.1111/j.1476-5381.1990.tb12048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: II. Calcium requirement. J Neurochem. 1984 May;42(5):1388–1394. doi: 10.1111/j.1471-4159.1984.tb02799.x. [DOI] [PubMed] [Google Scholar]
- Knepper S. M., Rutledge C. O. Effects of calcium depletion on norepinephrine- and A23187-induced stimulation of inositol phosphate formation. Biochem Pharmacol. 1987 Sep 15;36(18):3043–3050. doi: 10.1016/0006-2952(87)90222-x. [DOI] [PubMed] [Google Scholar]
- Komori S., Kawai M., Takewaki T., Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J Physiol. 1992 May;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondou H., Inagaki N., Fukui H., Koyama Y., Kanamura A., Wada H. Histamine-induced inositol phosphate accumulation in type-2 astrocytes. Biochem Biophys Res Commun. 1991 Jun 14;177(2):734–738. doi: 10.1016/0006-291x(91)91849-8. [DOI] [PubMed] [Google Scholar]
- Kunysz E. A., Michel A. D., Whiting R. L., Woods K. The human astrocytoma cell line 1321 N1 contains M2-glandular type muscarinic receptors linked to phosphoinositide turnover. Br J Pharmacol. 1989 Feb;96(2):271–278. doi: 10.1111/j.1476-5381.1989.tb11813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lucherini M. J., Gruenstein E. Histamine H1 receptors in UC-11MG astrocytes and their regulation of cytoplasmic Ca2+. Brain Res. 1992 Oct 2;592(1-2):193–201. doi: 10.1016/0006-8993(92)91676-6. [DOI] [PubMed] [Google Scholar]
- Medrano S., Gruenstein E., Dimlich R. V. Histamine stimulates glycogenolysis in human astrocytoma cells by increasing intracellular free calcium. Brain Res. 1992 Oct 2;592(1-2):202–207. doi: 10.1016/0006-8993(92)91677-7. [DOI] [PubMed] [Google Scholar]
- Michel M. C., Hanft G., Gross G. Alpha 1 B- but not alpha 1 A-adrenoceptors mediate inositol phosphate generation. Naunyn Schmiedebergs Arch Pharmacol. 1990 Apr;341(4):385–387. doi: 10.1007/BF00180666. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Atkinson B. Interaction of subtype-selective antagonists with alpha 1-adrenergic receptor-mediated second messenger responses in rat brain. Mol Pharmacol. 1991 Oct;40(4):523–530. [PubMed] [Google Scholar]
- Murphy S., Pearce B. Functional receptors for neurotransmitters on astroglial cells. Neuroscience. 1987 Aug;22(2):381–394. doi: 10.1016/0306-4522(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Nakahata N., Martin M. W., Hughes A. R., Hepler J. R., Harden T. K. H1-histamine receptors on human astrocytoma cells. Mol Pharmacol. 1986 Feb;29(2):188–195. [PubMed] [Google Scholar]
- Palacios J. M., Wamsley J. K., Kuhar M. J. GABA benzodiazepine and histamine-H1 receptors in the guinea pig cerebellum: effects of kainic acid injections studied by autoradiographic methods. Brain Res. 1981 Jun 9;214(1):155–162. doi: 10.1016/0006-8993(81)90447-9. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Moskowitz M. A., Reinhard J. F., Jr, Snyder S. H. Neurotransmitter receptor binding in bovine cerebral microvessels. Science. 1980 May 9;208(4444):610–612. doi: 10.1126/science.6102801. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Quach T. T., Duchemin A. M., Rose C., Schwartz J. C. 3H-Glycogen hydrolysis elicited by histamine in mouse brain slices: selective involvement of H1 receptors. Mol Pharmacol. 1980 May;17(3):301–308. [PubMed] [Google Scholar]
- Rodbard D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem. 1974 Oct;20(10):1255–1270. [PubMed] [Google Scholar]
- Schwartz J. C., Arrang J. M., Garbarg M., Pollard H., Ruat M. Histaminergic transmission in the mammalian brain. Physiol Rev. 1991 Jan;71(1):1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Summers R. J., McMartin L. R. Adrenoceptors and their second messenger systems. J Neurochem. 1993 Jan;60(1):10–23. doi: 10.1111/j.1471-4159.1993.tb05817.x. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., Lambrechts A. C., Tertoolen L. G., de Laat S. W., Moolenaar W. H. Regulation of phosphoinositide hydrolysis induced by histamine and guanine nucleotides in human HeLa carcinoma cells. Calcium and pH dependence and inhibitory role of protein kinase C. FEBS Lett. 1990 Jun 4;265(1-2):80–84. doi: 10.1016/0014-5793(90)80888-p. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., Tertoolen L. G., Lambrechts A. C., Remorie R., de Laat S. W., Moolenaar W. H. Histamine-H1-receptor-mediated phosphoinositide hydrolysis, Ca2+ signalling and membrane-potential oscillations in human HeLa carcinoma cells. Biochem J. 1990 Feb 15;266(1):235–243. doi: 10.1042/bj2660235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treherne J. M., Stern J. S., Flack W. J., Young J. M. Inhibition by cations of antagonist binding to histamine H1-receptors: differential effect of sodium ions on the binding of two radioligands. Br J Pharmacol. 1991 Jul;103(3):1745–1751. doi: 10.1111/j.1476-5381.1991.tb09857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Schmidt B. H., Sebben M., Kemp D. E., Bockaert J., Sladeczek F. Neurotransmitter-induced inositol phosphate formation in neurons in primary culture. J Neurochem. 1988 May;50(5):1425–1433. doi: 10.1111/j.1471-4159.1988.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Chen G., Miwa K., Suzuki H. Permeability and Mg2+ blockade of histamine-operated cation channel in endothelial cells of rat intrapulmonary artery. J Physiol. 1992 May;450:395–408. doi: 10.1113/jphysiol.1992.sp019133. [DOI] [PMC free article] [PubMed] [Google Scholar]


