Abstract
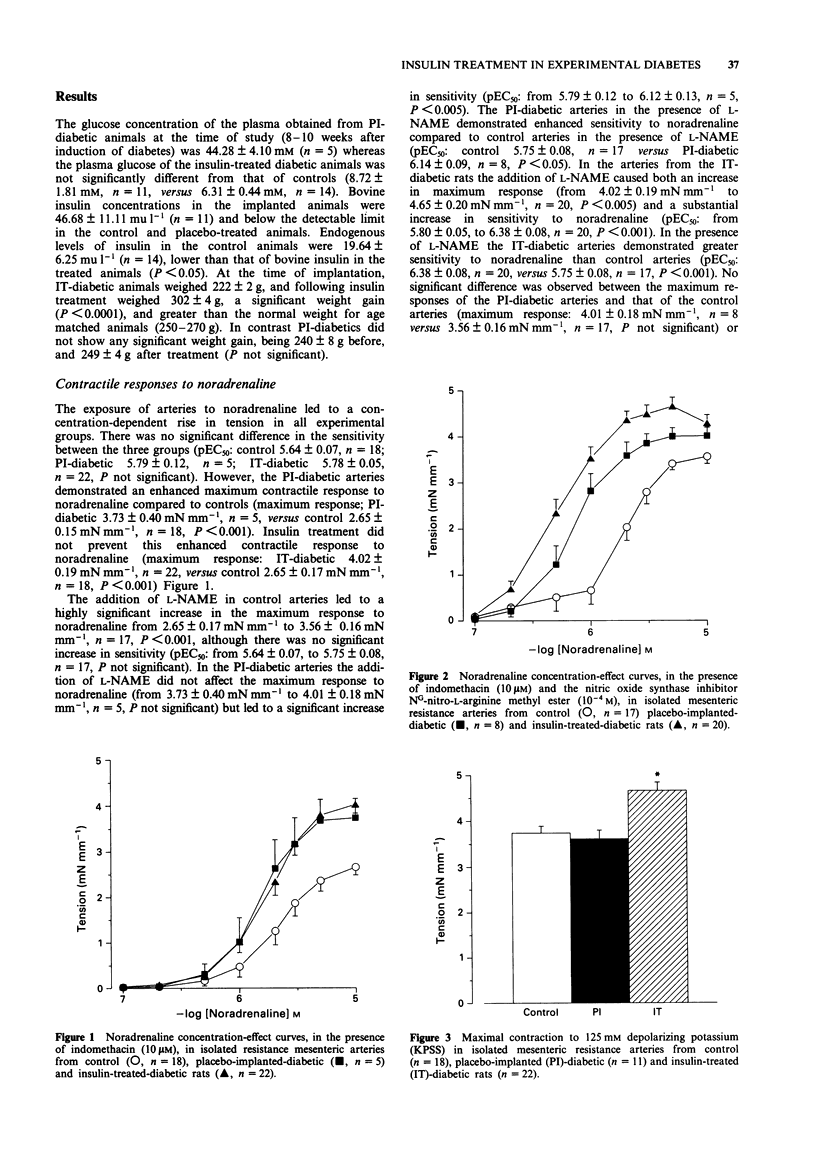
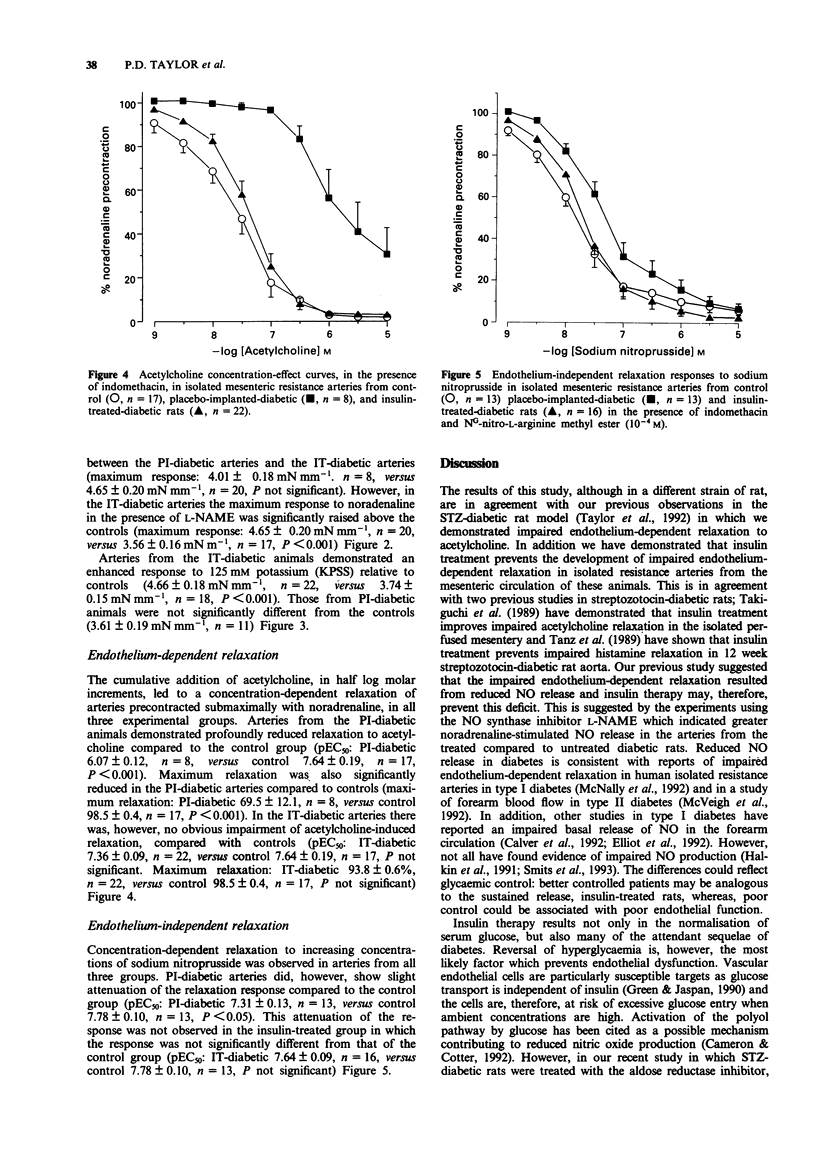
1. Streptozotocin-induced diabetic rats (Wistar) were implanted with sustained release insulin pellets (release rate = 4 u day-1) or with placebo pellets (palmitic acid) from the onset of glycosuria. 2. Noradrenaline sensitivity, endothelium-dependent relaxation to acetylcholine and endothelium-independent relaxation to sodium nitroprusside were assessed in mesenteric resistance arteries from the insulin-treated (IT) diabetic animals and compared to placebo-implanted (PI) diabetics and age-matched controls. 3. Arteries from PI-diabetic rats (8-10 weeks) demonstrated an enhanced maximal response to noradrenaline compared to controls, which was not prevented by insulin treatment (control 2.65 +/- 0.17 mN mm-1, n = 18 arteries versus PI-diabetic 3.73 +/- 0.40 mM mm-1, n = 5, P < 0.05; control versus IT-diabetic 4.02 +/- 0.19 mN mm-1, n = 22, P < 0.001). Sensitivity to noradrenaline was similar between the three groups. 4. In the presence of the nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME), IT and PI arteries were more sensitive to noradrenaline than control arteries (pEC50: control 5.75 +/- 0.08, n = 17, versus PI-diabetic 6.14 +/- 0.09, n = 8, P < 0.05; control versus IT-diabetic 6.38 +/- 0.08, n = 20, P < 0.001). 5. The maximum contractile response to depolarizing 125 mM K+ was significantly enhanced in IT-diabetic arteries but not PI-diabetic when compared to control arteries (maximum response: control 3.74 +/- 0.15 mN mm-1, n = 18, versus PI-diabetic 3.61 +/- 0.19 mN mm-1, n = 11, NS; control versus IT-diabetic 4.66 +/- 0.18 mN mm-1, n = 22, P < 0.001). 6. Endothelium-dependent relaxation to acetylcholine was profoundly impaired in the PI-diabetic arteries, but in the IT-diabetic arteries was not significantly different from controls (pEC50: control 7.64 +/- 0.19, n = 17, versus PI-diabetic 6.07 +/- 0.12, n = 8, P < 0.001; control versus IT-diabetic 7.36 +/- 0.09, n = 22, NS).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiru T., Kamata K., Miyata N., Kasuya Y. Differences in vascular responses to vasoactive agents of basilar artery and aorta from rabbits with alloxan-induced diabetes. Can J Physiol Pharmacol. 1990 Jul;68(7):882–888. doi: 10.1139/y90-134. [DOI] [PubMed] [Google Scholar]
- Agrawal D. K., McNeill J. H. Effect of diabetes on vascular smooth muscle function in normotensive and spontaneously hypertensive rat mesenteric artery. Can J Physiol Pharmacol. 1987 Nov;65(11):2274–2280. doi: 10.1139/y87-360. [DOI] [PubMed] [Google Scholar]
- Banskota N. K., Taub R., Zellner K., Olsen P., King G. L. Characterization of induction of protooncogene c-myc and cellular growth in human vascular smooth muscle cells by insulin and IGF-I. Diabetes. 1989 Jan;38(1):123–129. doi: 10.2337/diab.38.1.123. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991 Feb;87(2):432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver A., Collier J., Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992 Dec;90(6):2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A. Impaired contraction and relaxation in aorta from streptozotocin-diabetic rats: role of polyol pathway. Diabetologia. 1992 Nov;35(11):1011–1019. doi: 10.1007/BF02221675. [DOI] [PubMed] [Google Scholar]
- Chowienczyk P. J., Watts G. F., Cockcroft J. R., Ritter J. M. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet. 1992 Dec 12;340(8833):1430–1432. doi: 10.1016/0140-6736(92)92621-l. [DOI] [PubMed] [Google Scholar]
- Christlieb A. R., Janka H. U., Kraus B., Gleason R. E., Icasas-Cabral E. A., Aiello L. M., Cabral B. V., Solano A. Vascular reactivity to angiotensin II and to norepinephrine in diabetic subjects. Diabetes. 1976 Apr;25(4):268–274. doi: 10.2337/diab.25.4.268. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Tesfamariam B., Weisbrod R. M., Zitnay K. M. Adrenergic denervation in rabbits with diabetes mellitus. Am J Physiol. 1990 Jul;259(1 Pt 2):H55–H61. doi: 10.1152/ajpheart.1990.259.1.H55. [DOI] [PubMed] [Google Scholar]
- Creager M. A., Cooke J. P., Mendelsohn M. E., Gallagher S. J., Coleman S. M., Loscalzo J., Dzau V. J. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990 Jul;86(1):228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante W., Sen A. K., Sunahara F. A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988 Jun;94(2):463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler H. G., Blaschke T. F., Kraemer F. B., Ford G. A., Blöchl-Daum B., Hoffman B. B. Responsiveness of superficial hand veins to alpha-adrenoceptor agonists in insulin-dependent diabetic patients. Clin Sci (Lond) 1992 Feb;82(2):163–168. doi: 10.1042/cs0820163. [DOI] [PubMed] [Google Scholar]
- Gans R. O., Donker A. J. Insulin and blood pressure regulation. J Intern Med Suppl. 1991;735:49–64. [PubMed] [Google Scholar]
- Halkin A., Benjamin N., Doktor H. S., Todd S. D., Viberti G., Ritter J. M. Vascular responsiveness and cation exchange in insulin-dependent diabetes. Clin Sci (Lond) 1991 Aug;81(2):223–232. doi: 10.1042/cs0810223. [DOI] [PubMed] [Google Scholar]
- Harris K. H., MacLeod K. M. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur J Pharmacol. 1988 Aug 9;153(1):55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- Hodgson W. C., King R. G. Effects of glucose, insulin or aldose reductase inhibition on responses to endothelin-1 of aortic rings from streptozotocin-induced diabetic rats. Br J Pharmacol. 1992 Jul;106(3):644–649. doi: 10.1111/j.1476-5381.1992.tb14389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M., Cerami A., Bucala R. Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide. Role in the vascular and renal complications of diabetes mellitus. J Clin Invest. 1992 Sep;90(3):1110–1115. doi: 10.1172/JCI115928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Edwards J. C., Gruetter D. Y., Barry B. K., Gruetter C. A. Possible involvement of S-nitrosothiols in the activation of guanylate cyclase by nitroso compounds. FEBS Lett. 1980 Feb 11;110(2):275–278. doi: 10.1016/0014-5793(80)80091-3. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Plane F., Bruckdorfer K. R. Native and oxidized low-density lipoproteins have different inhibitory effects on endothelium-derived relaxing factor in the rabbit aorta. Br J Pharmacol. 1990 May;100(1):21–26. doi: 10.1111/j.1476-5381.1990.tb12045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989 Jun;97(2):614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Doherty J. U., Faillace R., Maekawa K., Arnold S., Gavras H., Hood W. B., Jr Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J Clin Invest. 1982 Jun;69(6):1321–1336. doi: 10.1172/JCI110572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod K. M., McNeill J. H. The influence of chronic experimental diabetes on contractile responses of rat isolated blood vessels. Can J Physiol Pharmacol. 1985 Jan;63(1):52–57. doi: 10.1139/y85-009. [DOI] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G. Impairment of endothelium-dependent dilatation of cerebral arterioles during diabetes mellitus. Am J Physiol. 1989 Mar;256(3 Pt 2):H621–H625. doi: 10.1152/ajpheart.1989.256.3.H621. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G., Simmons L. K., Sharpe G. M. Mechanism of impaired responses of cerebral arterioles during diabetes mellitus. Am J Physiol. 1991 Feb;260(2 Pt 2):H319–H326. doi: 10.1152/ajpheart.1991.260.2.H319. [DOI] [PubMed] [Google Scholar]
- McVeigh G. E., Brennan G. M., Johnston G. D., McDermott B. J., McGrath L. T., Henry W. R., Andrews J. W., Hayes J. R. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993 Jan;36(1):33–38. doi: 10.1007/BF00399090. [DOI] [PubMed] [Google Scholar]
- McVeigh G. E., Brennan G. M., Johnston G. D., McDermott B. J., McGrath L. T., Henry W. R., Andrews J. W., Hayes J. R. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992 Aug;35(8):771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- Moorhouse J. A., Carter S. A., Doupe J. Vascular responses in diabetic peripheral neuropathy. Br Med J. 1966 Apr 9;1(5492):883–888. doi: 10.1136/bmj.1.5492.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Pfaffman M. A., Ball C. R., Darby A., Hilman R. Insulin reversal of diabetes-induced inhibition of vascular contractility in the rat. Am J Physiol. 1982 Apr;242(4):H490–H495. doi: 10.1152/ajpheart.1982.242.4.H490. [DOI] [PubMed] [Google Scholar]
- Pieper G. M., Gross G. J. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1988 Oct;255(4 Pt 2):H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Sato N., Hashimoto H., Takiguchi Y., Nakashima M. Altered responsiveness to sympathetic nerve stimulation and agonists of isolated left atria of diabetic rats: no evidence for involvement of hypothyroidism. J Pharmacol Exp Ther. 1989 Jan;248(1):367–371. [PubMed] [Google Scholar]
- Scarborough N. L., Carrier G. O. Increased alpha 2-adrenoreceptor mediated vascular contraction in diabetic rats. J Auton Pharmacol. 1983 Sep;3(3):177–183. doi: 10.1111/j.1474-8673.1983.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Scott A. R., Bennett T., MacDonald I. A. Effects of hyperinsulinaemia on the cardiovascular responses to graded hypovolaemia in normal and diabetic subjects. Clin Sci (Lond) 1988 Jul;75(1):85–92. doi: 10.1042/cs0750085. [DOI] [PubMed] [Google Scholar]
- Smits P., Kapma J. A., Jacobs M. C., Lutterman J., Thien T. Endothelium-dependent vascular relaxation in patients with type I diabetes. Diabetes. 1993 Jan;42(1):148–153. doi: 10.2337/diab.42.1.148. [DOI] [PubMed] [Google Scholar]
- Sosenko J. M., Breslow J. L., Miettinen O. S., Gabbay K. H. Hyperglycemia and plasma lipid levels: a prospective study of young insulin-dependent diabetic patients. N Engl J Med. 1980 Mar 20;302(12):650–654. doi: 10.1056/NEJM198003203021202. [DOI] [PubMed] [Google Scholar]
- Standley P. R., Zhang F., Ram J. L., Zemel M. B., Sowers J. R. Insulin attenuates vasopressin-induced calcium transients and a voltage-dependent calcium response in rat vascular smooth muscle cells. J Clin Invest. 1991 Oct;88(4):1230–1236. doi: 10.1172/JCI115426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolar M. W. Atherosclerosis in diabetes: the role of hyperinsulinemia. Metabolism. 1988 Feb;37(2 Suppl 1):1–9. doi: 10.1016/0026-0495(88)90180-1. [DOI] [PubMed] [Google Scholar]
- Tagami S., Kondo T., Yoshida K., Hirokawa J., Ohtsuka Y., Kawakami Y. Effect of insulin on impaired antioxidant activities in aortic endothelial cells from diabetic rabbits. Metabolism. 1992 Oct;41(10):1053–1058. doi: 10.1016/0026-0495(92)90285-i. [DOI] [PubMed] [Google Scholar]
- Takiguchi Y., Satoh N., Hashimoto H., Nakashima M. Reversal effect of thyroxine on altered vascular reactivity in diabetic rats. J Cardiovasc Pharmacol. 1989 Apr;13(4):520–524. [PubMed] [Google Scholar]
- Tanz R. D., Chang K. S., Weller T. S. Histamine relaxation of aortic rings from diabetic rats. Agents Actions. 1989 Aug;28(1-2):1–8. doi: 10.1007/BF02022973. [DOI] [PubMed] [Google Scholar]
- Taylor P. D., McCarthy A. L., Thomas C. R., Poston L. Endothelium-dependent relaxation and noradrenaline sensitivity in mesenteric resistance arteries of streptozotocin-induced diabetic rats. Br J Pharmacol. 1992 Oct;107(2):393–399. doi: 10.1111/j.1476-5381.1992.tb12757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. D., Wickenden A. D., Mirrlees D. J., Poston L. Endothelial function in the isolated perfused mesentery and aortae of rats with streptozotocin-induced diabetes: effect of treatment with the aldose reductase inhibitor, ponalrestat. Br J Pharmacol. 1994 Jan;111(1):42–48. doi: 10.1111/j.1476-5381.1994.tb14021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Deykin D., Cohen R. A. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990 Mar;85(3):929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Cohen R. A. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992 Aug;263(2 Pt 2):H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- Wambach G. K., Liu D. M. Insulin attenuates vasoconstriction induced by noradrenaline, serotonin and potassium chloride in rat mesenteric resistance arteries. J Hypertens Suppl. 1991 Dec;9(6):S198–S199. [PubMed] [Google Scholar]
- Wang P. Y. Palmitic acid as an excipient in implants for sustained release of insulin. Biomaterials. 1991 Jan;12(1):57–62. doi: 10.1016/0142-9612(91)90133-u. [DOI] [PubMed] [Google Scholar]
- White R. E., Carrier G. O. Enhanced vascular alpha-adrenergic neuroeffector system in diabetes: importance of calcium. Am J Physiol. 1988 Nov;255(5 Pt 2):H1036–H1042. doi: 10.1152/ajpheart.1988.255.5.H1036. [DOI] [PubMed] [Google Scholar]
- Wong K. K., Tzeng S. F. Norepinephrine-induced contractile responses in isolated rat aortae from different duration of diabetes. Artery. 1992;19(1):1–13. [PubMed] [Google Scholar]
- Yagi S., Takata S., Kiyokawa H., Yamamoto M., Noto Y., Ikeda T., Hattori N. Effects of insulin on vasoconstrictive responses to norepinephrine and angiotensin II in rabbit femoral artery and vein. Diabetes. 1988 Aug;37(8):1064–1067. doi: 10.2337/diab.37.8.1064. [DOI] [PubMed] [Google Scholar]
- Zeiher A. M., Drexler H., Wollschläger H., Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991 Feb;83(2):391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]


