Abstract
1. The present experiments were undertaken to determine the mechanism(s) of hyperkalaemia caused by nafamostat mesilate (NM), a serine-protease inhibitor. 2. We investigated the effects of luminal addition of two metabolites of NM, p-guanidinobenzoic acid (PGBA) and 6-amidino-2-naphthol (AN), on Na+ and K+ transport properties of the collecting duct (CD) cell in the isolated perfused cortical collecting duct (CCD) from rabbit kidneys, because these metabolites, but not NM, were mainly excreted into the urine. 3. Addition of PGBA at 10(-5) and 10(-4) M in the lumen resulted in a hyperpolarization of VA in parallel with increases in transepithelial resistance (RT) and fractional apical membrane resistance (fRA). PGBA added to the luminal perfusate at 10(-5) and 10(-4) M changed VA, RT and fRA in a dose-dependent manner. These effects were completely inhibited by pretreatment with luminal amiloride (50 microM). PGBA at 10(-6) M in the lumen had no effect on the electrical parameters. 4. Luminal addition of AN at 10(-4) M also caused the apical membrane to hyperpolarize in parallel with increases in RT and fRA. These effects were also completely inhibited by pretreatment with luminal amiloride (50 microM). AN at 10(-5) M in the lumen had no effect on the electrical parameters. 5. We conclude that two metabolites of NM, PGBA and AN, act on the apical membrane of the CD cell and inhibit the amiloride-sensitive Na+ conductance, resulting in an inhibition of K+ secretion. This direct action of these metabolites, rather than NM, on the CCD might contribute to the NM-induced hyperkalaemia.
Full text
PDF
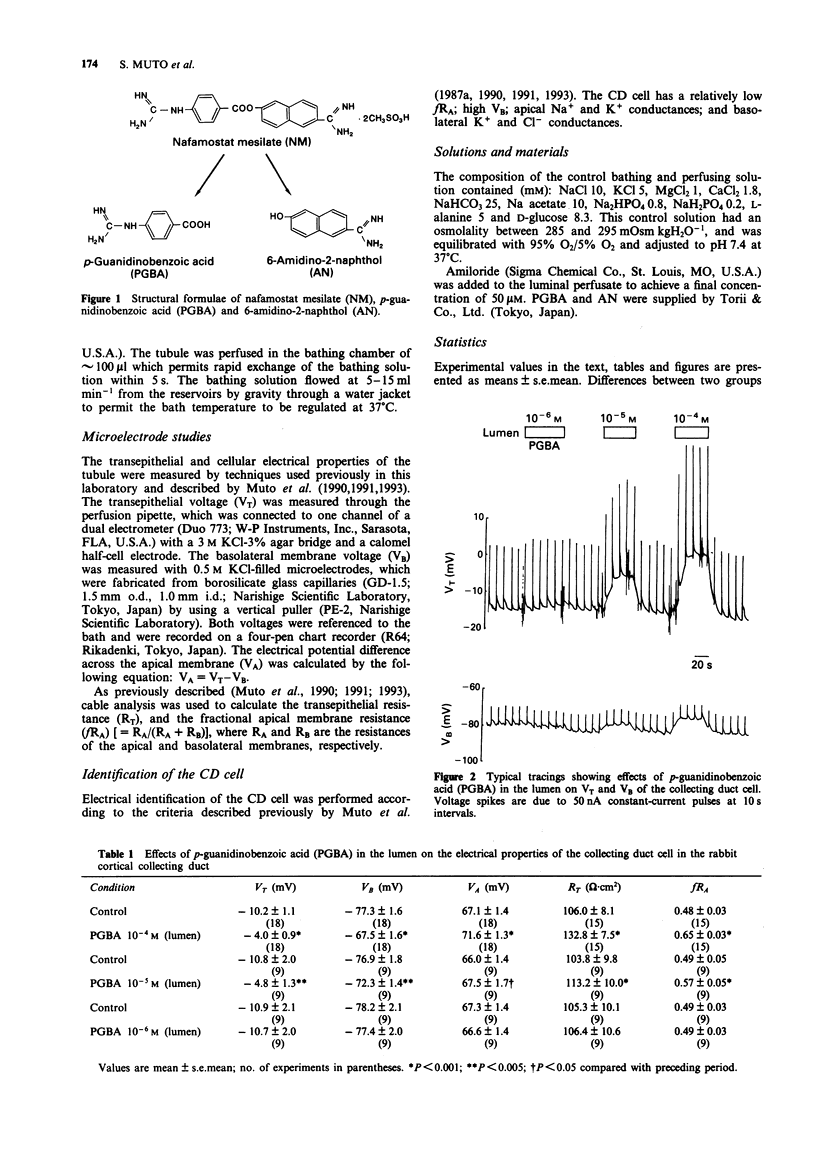
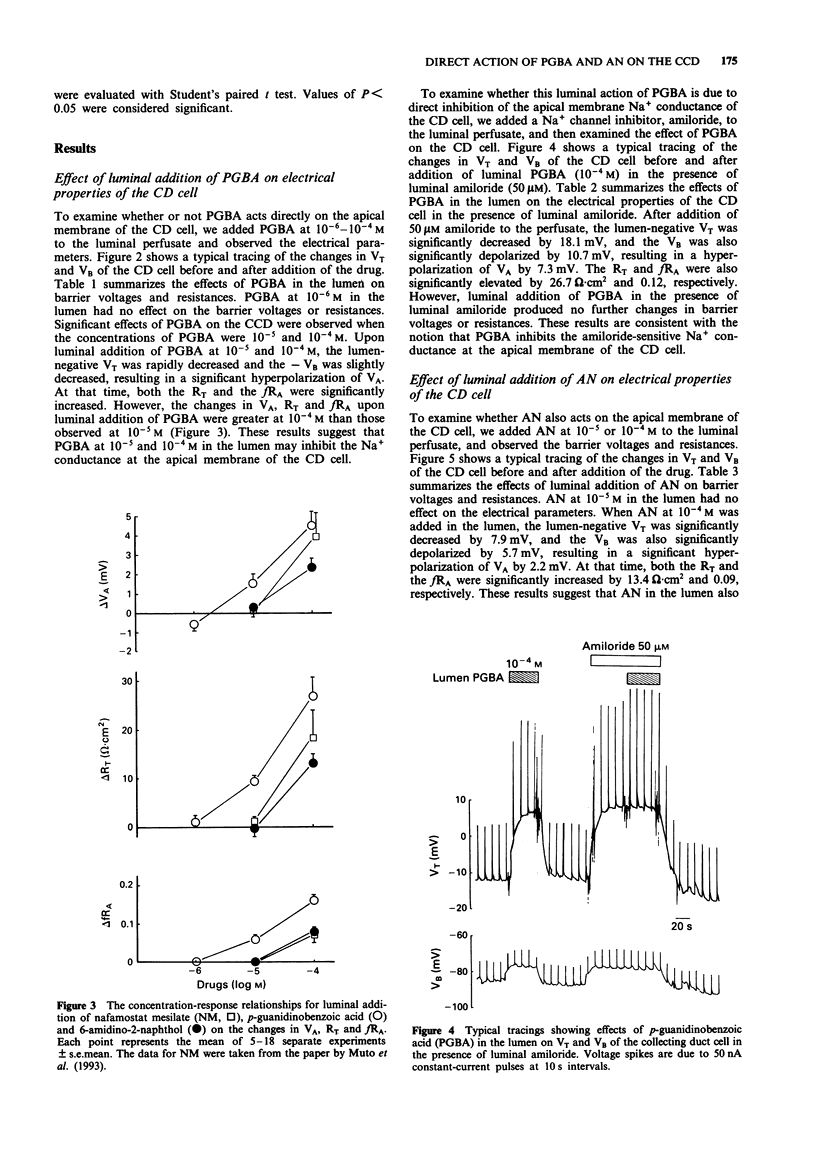
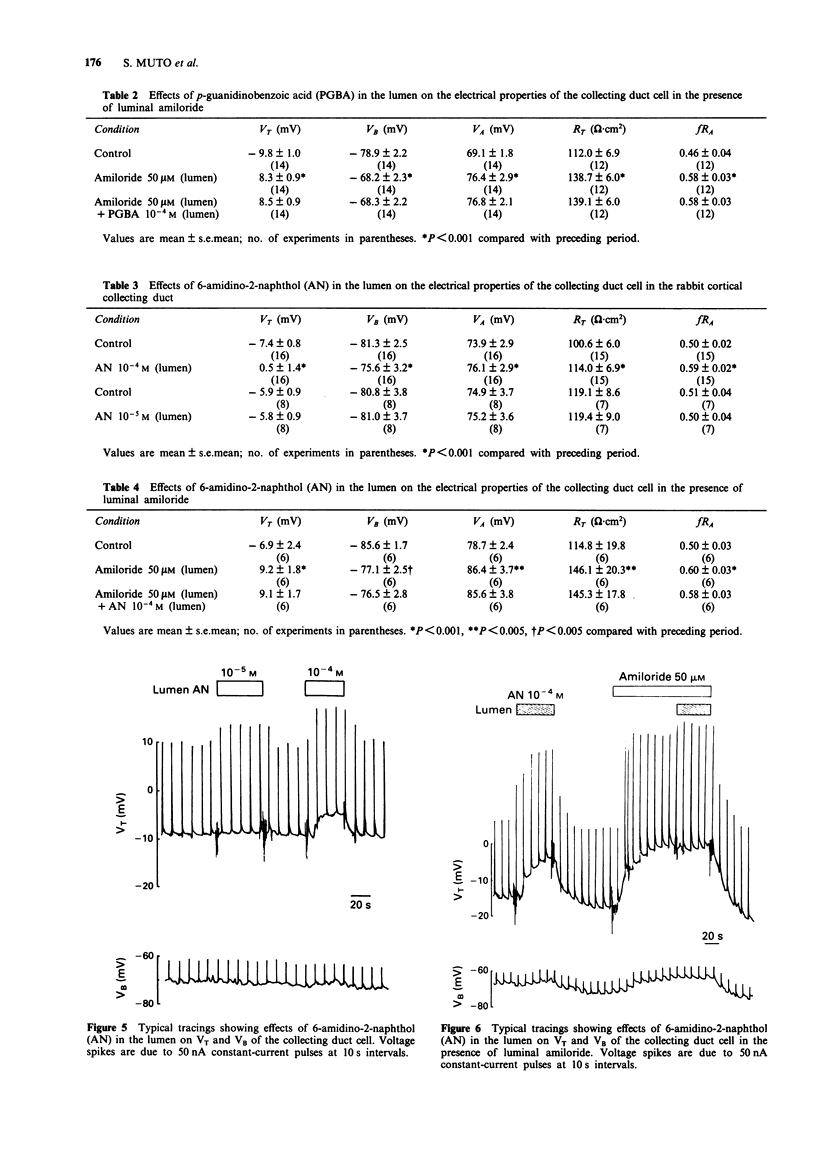


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama T., Ino Y., Ozeki M., Oda M., Sato T., Koshiyama Y., Suzuki S., Fujita M. Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments. Jpn J Pharmacol. 1984 Jul;35(3):203–227. doi: 10.1254/jjp.35.203. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Okutome T., Nakayama T., Yaegashi T., Matsui R., Nunomura S., Kurumi M., Sakurai Y., Fujii S. Synthesis and structure-activity study of protease inhibitors. IV. Amidinonaphthols and related acyl derivatives. Chem Pharm Bull (Tokyo) 1985 Apr;33(4):1458–1471. doi: 10.1248/cpb.33.1458. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Hitomi Y., Ikari N., Fujii S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Haemostasis. 1985;15(3):164–168. doi: 10.1159/000215139. [DOI] [PubMed] [Google Scholar]
- Iwaki M., Ino Y., Motoyoshi A., Ozeki M., Sato T., Kurumi M., Aoyama T. Pharmacological studies of FUT-175, nafamostat mesilate. V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats. Jpn J Pharmacol. 1986 Jun;41(2):155–162. doi: 10.1254/jjp.41.155. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Muto S., Furuya H., Tabei K., Asano Y. Site and mechanism of action of epidermal growth factor in rabbit cortical collecting duct. Am J Physiol. 1991 Feb;260(2 Pt 2):F163–F169. doi: 10.1152/ajprenal.1991.260.2.F163. [DOI] [PubMed] [Google Scholar]
- Muto S., Giebisch G., Sansom S. An acute increase of peritubular K stimulates K transport through cell pathways of CCT. Am J Physiol. 1988 Jul;255(1 Pt 2):F108–F114. doi: 10.1152/ajprenal.1988.255.1.F108. [DOI] [PubMed] [Google Scholar]
- Muto S., Giebisch G., Sansom S. Effects of adrenalectomy on CCD: evidence for differential response of two cell types. Am J Physiol. 1987 Oct;253(4 Pt 2):F742–F752. doi: 10.1152/ajprenal.1987.253.4.F742. [DOI] [PubMed] [Google Scholar]
- Muto S., Imai M., Asano Y. Effect of nafamostat mesilate on Na+ and K+ transport properties in the rabbit cortical collecting duct. Br J Pharmacol. 1993 Jul;109(3):673–678. doi: 10.1111/j.1476-5381.1993.tb13626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Sansom S., Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting ducts from adrenalectomized rabbits. J Clin Invest. 1988 Feb;81(2):376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Yasoshima K., Yoshitomi K., Imai M., Asano Y. Electrophysiological identification of alpha- and beta-intercalated cells and their distribution along the rabbit distal nephron segments. J Clin Invest. 1990 Dec;86(6):1829–1839. doi: 10.1172/JCI114913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil R. G., Sansom S. C. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol. 1984;82(3):281–295. doi: 10.1007/BF01871637. [DOI] [PubMed] [Google Scholar]
- Ohtake Y., Hirasawa H., Sugai T., Oda S., Shiga H., Matsuda K., Kitamura N. Nafamostat mesylate as anticoagulant in continuous hemofiltration and continuous hemodiafiltration. Contrib Nephrol. 1991;93:215–217. doi: 10.1159/000420222. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Marukawa S., Hayami H., Ozaki K., Ishida H., Kono K. [Effect of nafamostat mesilate on the renin-aldosterone system]. Masui. 1992 Mar;41(3):326–330. [PubMed] [Google Scholar]
- Sansom S. C., Agulian S., Muto S., Illig V., Giebisch G. K activity of CCD principal cells from normal and DOCA-treated rabbits. Am J Physiol. 1989 Jan;256(1 Pt 2):F136–F142. doi: 10.1152/ajprenal.1989.256.1.F136. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., O'Neil R. G. Effects of mineralocorticoids on transport properties of cortical collecting duct basolateral membrane. Am J Physiol. 1986 Oct;251(4 Pt 2):F743–F757. doi: 10.1152/ajprenal.1986.251.4.F743. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., O'Neil R. G. Mineralocorticoid regulation of apical cell membrane Na+ and K+ transport of the cortical collecting duct. Am J Physiol. 1985 Jun;248(6 Pt 2):F858–F868. doi: 10.1152/ajprenal.1985.248.6.F858. [DOI] [PubMed] [Google Scholar]
- Yamazaki Z., Hiraishi M., Kanai F., Takahama T., Idezuki Y., Inoue N. Pharmacodynamics of FUT-175 anticoagulant in adsorbent plasma perfusion. ASAIO Trans. 1989 Jul-Sep;35(3):567–569. doi: 10.1097/00002480-198907000-00128. [DOI] [PubMed] [Google Scholar]
- Yang H., Henkin J., Kim K. H., Greer J. Selective inhibition of urokinase by substituted phenylguanidines: quantitative structure-activity relationship analyses. J Med Chem. 1990 Nov;33(11):2956–2961. doi: 10.1021/jm00173a008. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Murakami M., Furukawa Y., Kato H., Takemura S., Kondo M. Effects of FUT-175, a new synthetic protease inhibitor on endotoxin-induced disseminated intravascular coagulation in rats. Haemostasis. 1983;13(6):374–378. doi: 10.1159/000214825. [DOI] [PubMed] [Google Scholar]


