Abstract
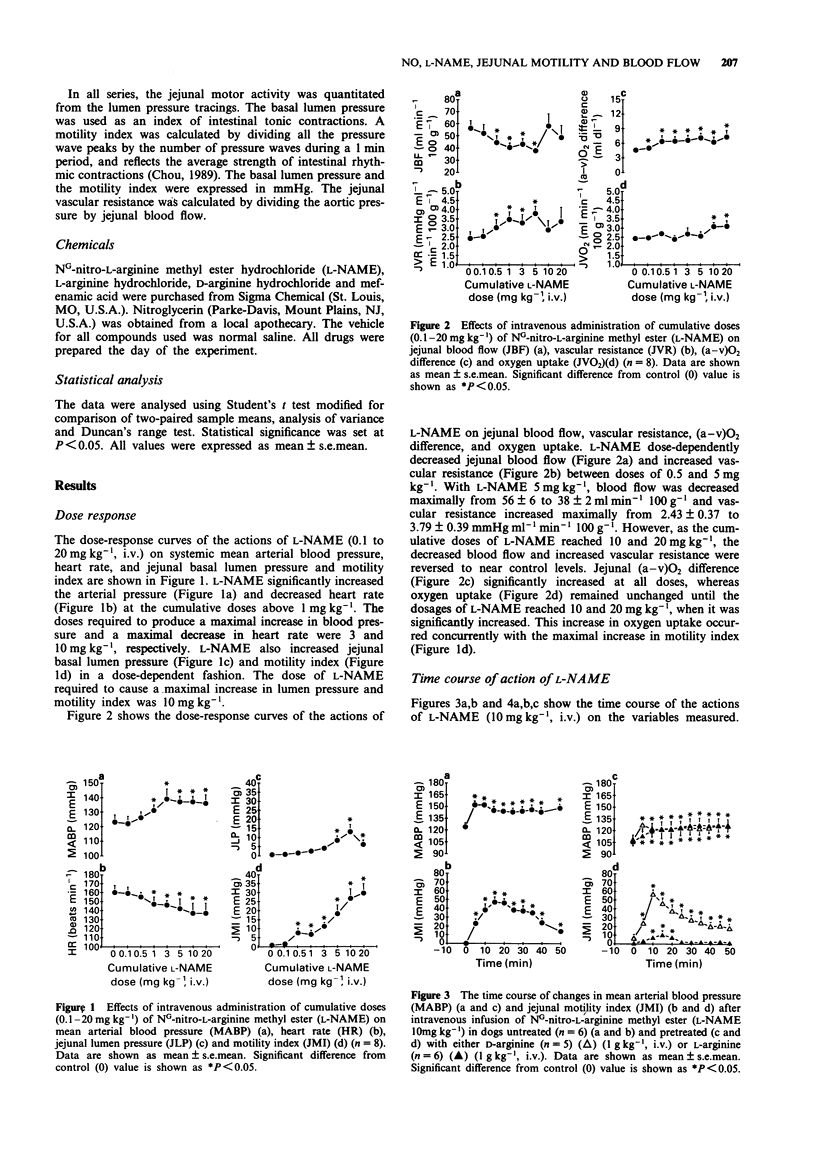
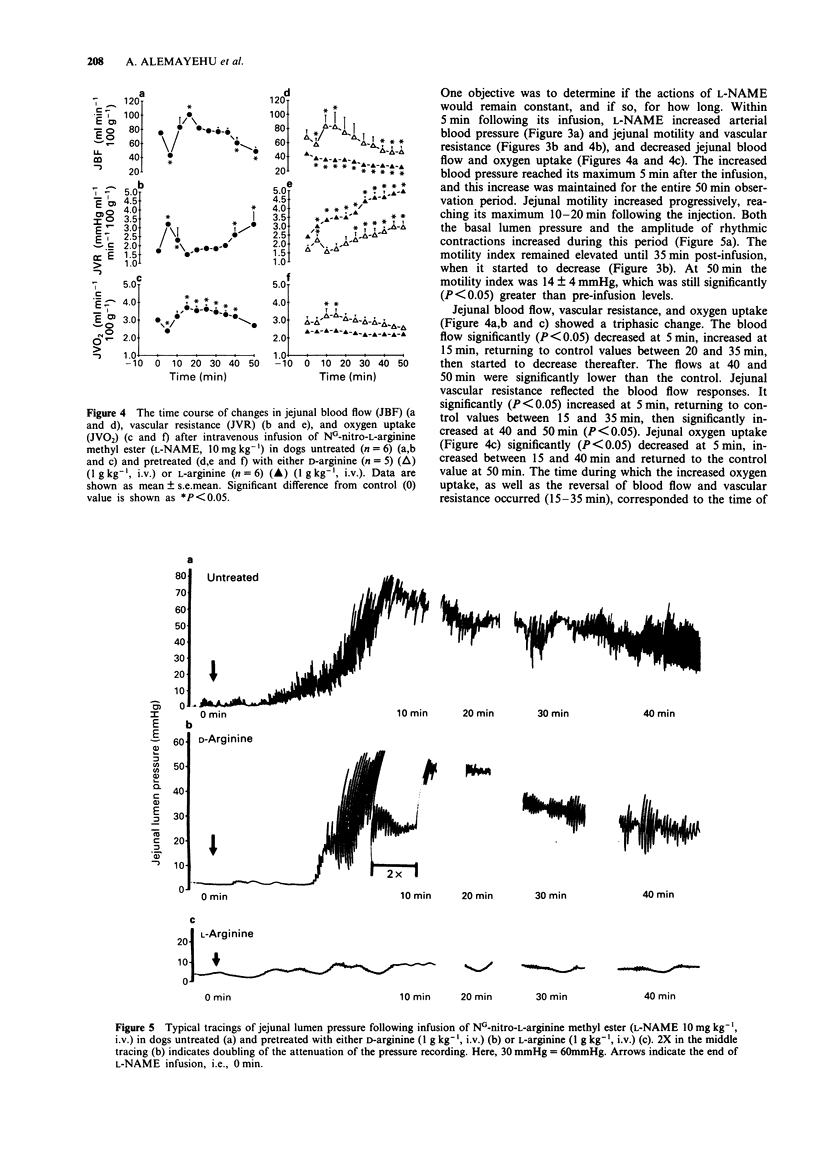
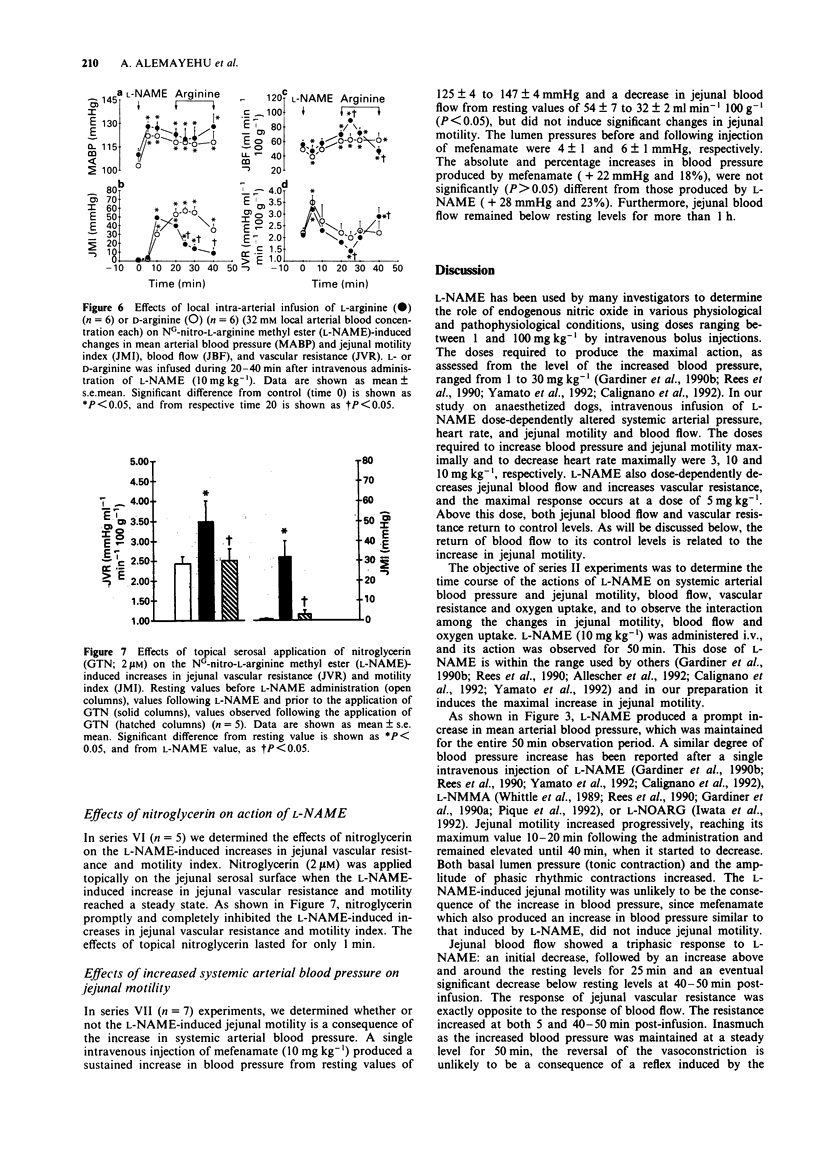
1. The effects of the inhibitor of nitric oxide (NO) synthesis, NG-nitro-L-arginine methyl ester (L-NAME), on systemic arterial blood pressure and jejunal motility, blood flow, and oxygen uptake have been investigated in anaesthetized dogs. 2. L-NAME (cumulative doses of 0.1-20 mg kg-1, i.v.) dose-dependently increased blood pressure and jejunal motility and decreased heart rate. The maximal response of these three variables occurred at doses, 3, 10 and 10 mg kg-1, respectively. L-NAME (cumulative doses of 0.5-5 mg kg-1) also dose-dependently induced jejunal vasoconstriction. The jejunal vascular resistance returned to control values as the cumulative doses reached 10 and 20 mg kg-1, which corresponded to the maximal increase in jejunal motility. 3. A single intravenous injection of L-NAME (10 mg kg-1) produced a prompt increase in blood pressure, which lasted for at least 50 min. 4. L-NAME (10 mg kg-1) produced a progressive rise in jejunal motility reaching its maximum (47 +/- 6 mmHg) 15 min after the administration, and lasting for 40-50 min. Both the basal lumen pressure and the amplitude of rhythmic contractions increased during this period. 5. L-NAME (10 mg kg-1) produced a triphasic change in jejunal vascular resistance and blood flow measured by timed collection of venous outflow. The blood flow decreased initially (-43% at 5 min), increased (+35%) and returned to control value between 15 and 35 min, then decreased (-35%) 40-50 min post-infusion. Jejunal vascular resistance reflected the blood flow response (+88% at both 5 and 50 min).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allescher H. D., Tougas G., Vergara P., Lu S., Daniel E. E. Nitric oxide as a putative nonadrenergic noncholinergic inhibitory transmitter in the canine pylorus in vivo. Am J Physiol. 1992 Apr;262(4 Pt 1):G695–G702. doi: 10.1152/ajpgi.1992.262.4.G695. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Calignano A., Whittle B. J., Di Rosa M., Moncada S. Involvement of endogenous nitric oxide in the regulation of rat intestinal motility in vivo. Eur J Pharmacol. 1992 Dec 15;229(2-3):273–276. doi: 10.1016/0014-2999(92)90567-n. [DOI] [PubMed] [Google Scholar]
- Chou C. C., Alemayehu A., Mangino M. J. Prostanoids in regulation of postprandial jejunal hyperemia and oxygen uptake. Am J Physiol. 1989 Nov;257(5 Pt 1):G798–G808. doi: 10.1152/ajpgi.1989.257.5.G798. [DOI] [PubMed] [Google Scholar]
- Dalziel H. H., Thornbury K. D., Ward S. M., Sanders K. M. Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol. 1991 May;260(5 Pt 1):G789–G792. doi: 10.1152/ajpgi.1991.260.5.G789. [DOI] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Frohlich E. D., Chou C., Champion P. K., Jr, Sytsma J., Anderson R. Effects of glyceryl trinitrate and pentaerythritol tetranitrate on forelimb, renal, mesenteric, and total peripheral resistances. Am Heart J. 1965 Nov;70(5):657–664. doi: 10.1016/0002-8703(65)90394-7. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata F., Ishii T., Kanada A., Yamano N., Kataoka T., Takeuchi T., Yagasaki O. Essential role of nitric oxide in descending inhibition in the rat proximal colon. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1400–1406. doi: 10.1016/0006-291x(90)91605-r. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Iwata F., Joh T., Kawai T., Itoh M. Role of EDRF in splanchnic blood flow of normal and chronic portal hypertensive rats. Am J Physiol. 1992 Aug;263(2 Pt 1):G149–G154. doi: 10.1152/ajpgi.1992.263.2.G149. [DOI] [PubMed] [Google Scholar]
- Jones L. F., Brody M. J. Coronary blood flow in rats is dependent on the release of vascular nitric oxide. J Pharmacol Exp Ther. 1992 Feb;260(2):627–631. [PubMed] [Google Scholar]
- Knudsen M. A., Svane D., Tøttrup A. Action profiles of nitric oxide, S-nitroso-L-cysteine, SNP, and NANC responses in opossum lower esophageal sphincter. Am J Physiol. 1992 May;262(5 Pt 1):G840–G846. doi: 10.1152/ajpgi.1992.262.5.G840. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Barrowman J. A., Harper S. L., Granger D. N. Relations among canine intestinal motility, blood flow, and oxygenation. Am J Physiol. 1986 Jul;251(1 Pt 1):G25–G33. doi: 10.1152/ajpgi.1986.251.1.G25. [DOI] [PubMed] [Google Scholar]
- Mangino M. J., Chou C. C. Thromboxane synthesis inhibitors and postprandial jejunal capillary exchange capacity. Am J Physiol. 1988 May;254(5 Pt 1):G695–G701. doi: 10.1152/ajpgi.1988.254.5.G695. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension. 1988 Oct;12(4):365–372. doi: 10.1161/01.hyp.12.4.365. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pique J. M., Esplugues J. V., Whittle B. J. Endogenous nitric oxide as a mediator of gastric mucosal vasodilatation during acid secretion. Gastroenterology. 1992 Jan;102(1):168–174. doi: 10.1016/0016-5085(92)91797-8. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992 Mar;262(3 Pt 1):G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Shepherd A. P., Burgar C. G. A solid-state arteriovenous oxygen difference analyzer for flowing whole blood. Am J Physiol. 1977 Apr;232(4):H437–H440. doi: 10.1152/ajpheart.1977.232.4.H437. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Thornbury K. D., Westfall D. P., Sanders K. M. Nonadrenergic, noncholinergic inhibition and rebound excitation in canine colon depend on nitric oxide. Am J Physiol. 1992 Feb;262(2 Pt 1):G237–G243. doi: 10.1152/ajpgi.1992.262.2.G237. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato S., Spechler S. J., Goyal R. K. Role of nitric oxide in esophageal peristalsis in the opossum. Gastroenterology. 1992 Jul;103(1):197–204. doi: 10.1016/0016-5085(92)91113-i. [DOI] [PubMed] [Google Scholar]


