Abstract
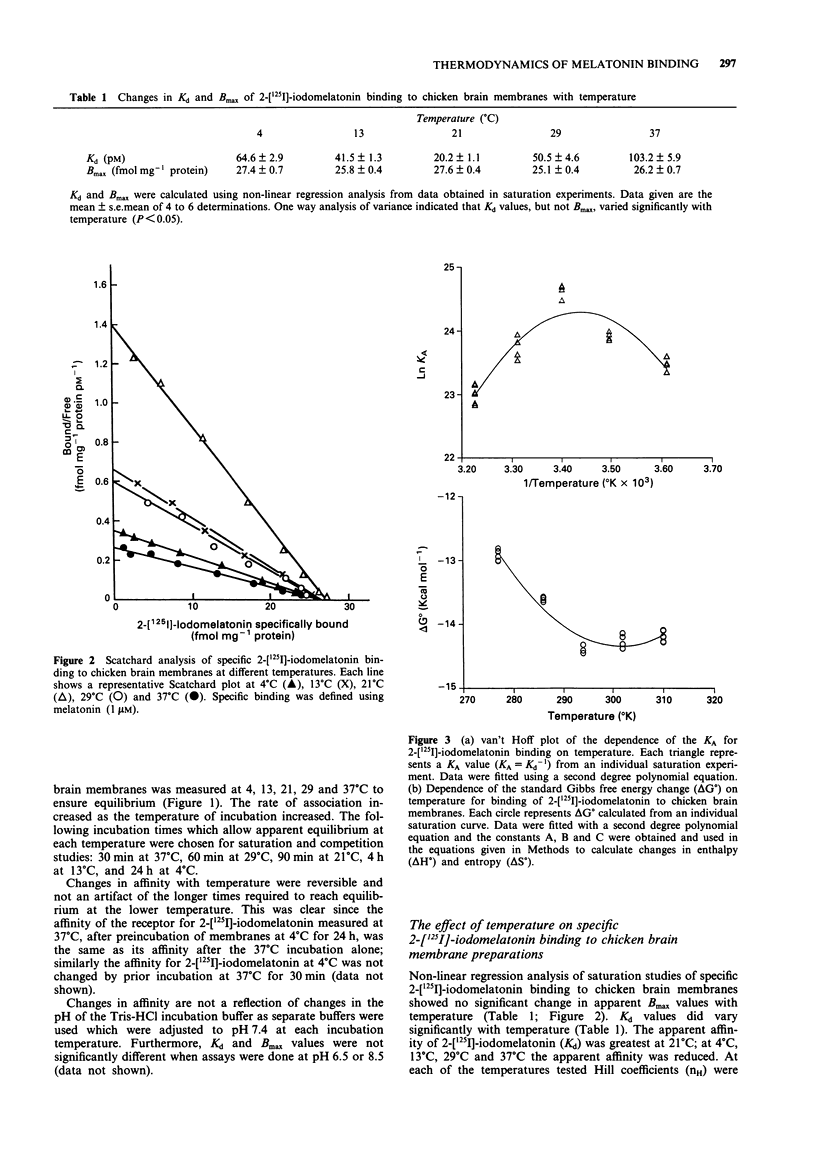
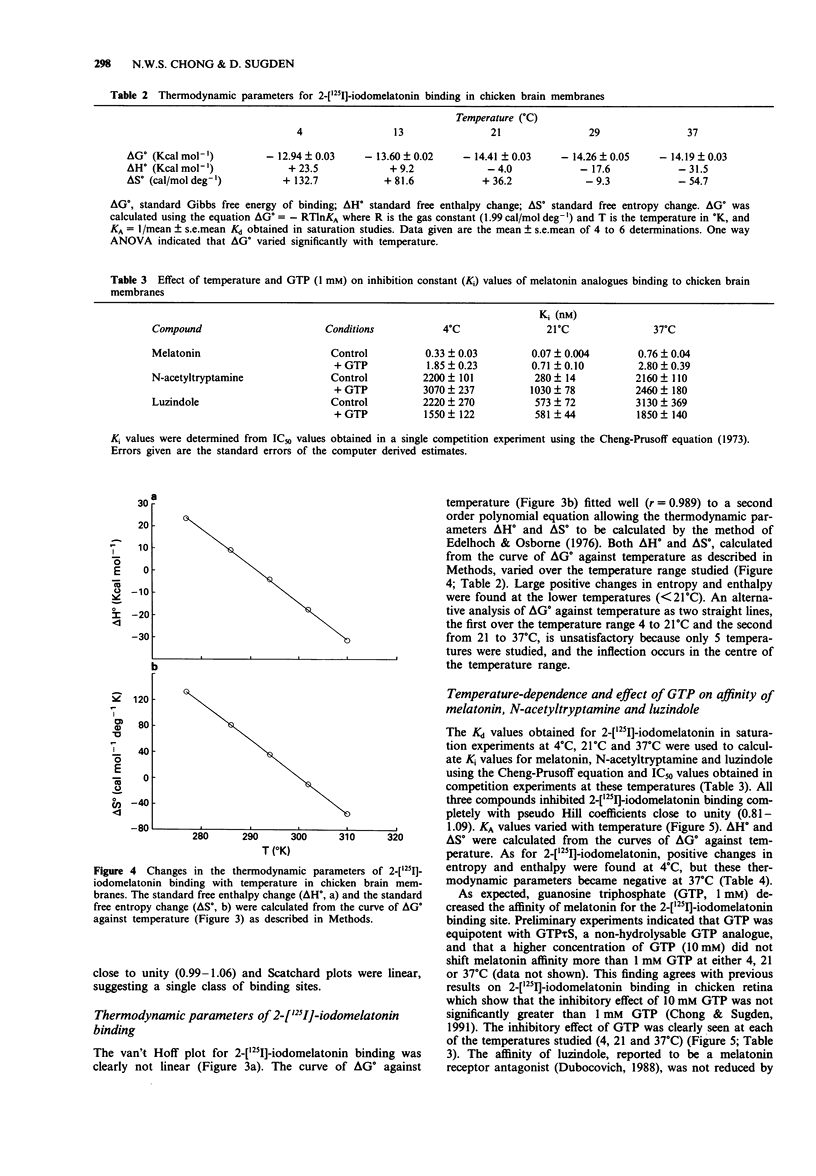
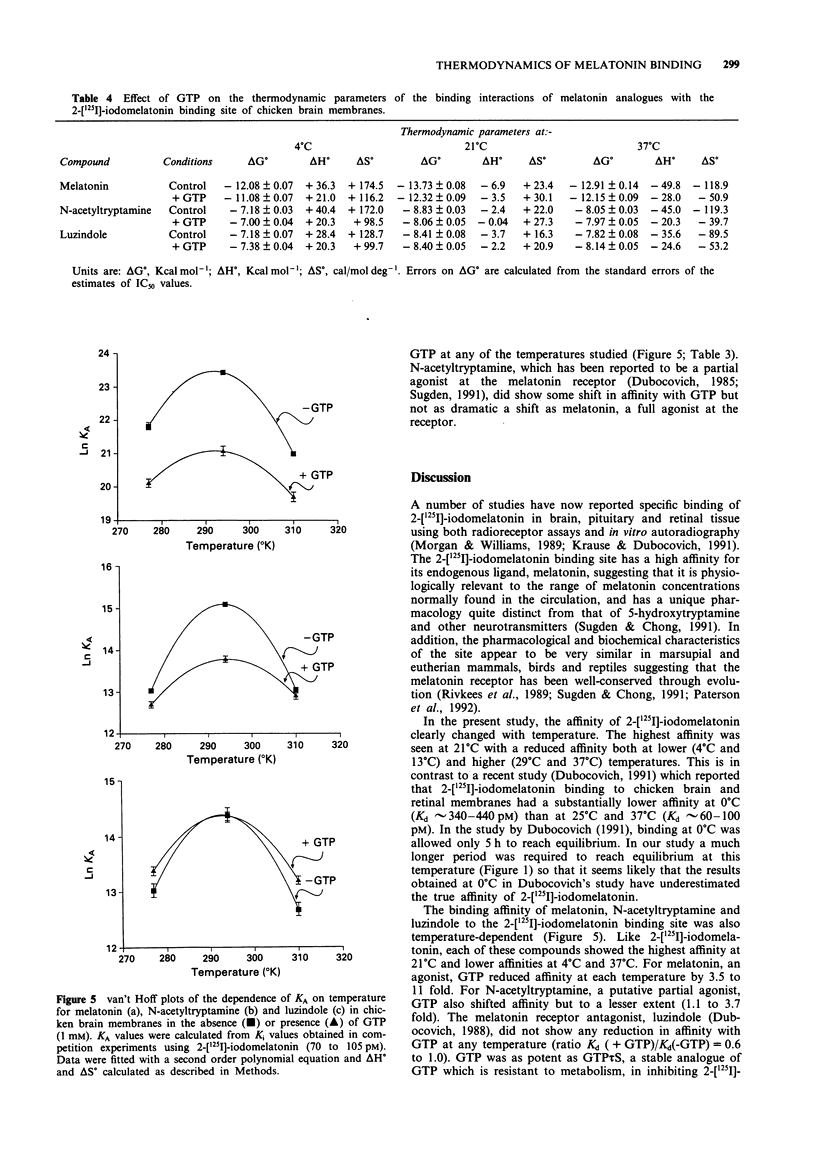
1. The binding of 2-[125I]-iodomelatonin to chicken brain membranes, and the inhibition of binding by melatonin, N-acetyltryptamine and luzindole, were examined at temperatures between 4 degrees C and 37 degrees C. 2. At all temperatures studied, the binding affinity (Kd or Ki) for 2-[125I]-iodomelatonin, melatonin (both agonists) and, to a lesser extent, N-acetyltryptamine (a partial agonist) was reduced by inclusion of guanosine triphosphate (GTP, 1 mM) in the assay. GTP did not affect the Ki for luzindole, a melatonin receptor antagonist. 3. The maximal density of binding sites (Bmax) was not affected by temperature but the Kd showed a peak at 21 degrees C with lower values at both higher and lower temperatures giving curvilinear van't Hoff plots (lnKA vs l/temperature). 4. Derived changes in entropy (delta S degree) and enthalpy (delta H degree) of binding for all of the melatonin ligands decreased as temperature increased. 5. The affinity, and thus the free energy of binding, delta G degree, of these ligands at the melatonin receptor have identical values at several temperatures yet at these temperatures delta S degree and delta H degree were very different, implying that more than one intermolecular force must be involved in the binding of ligand and receptor. 6. Conceivably, the large positive delta S degree observed at low temperatures, perhaps as a result of hydrophobic interactions, is compensated by a corresponding, but opposite, change in enthalpy at higher temperatures. However, it is not clear what type of binding force(s) would show such a temperature-dependence. 7. These studies suggest that caution must be exercised in the molecular interpretation of derived measures of delta S degree and delta H degree obtained from direct measurements of delta G degree.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartness T. J., Goldman B. D. Mammalian pineal melatonin: a clock for all seasons. Experientia. 1989 Oct 15;45(10):939–945. doi: 10.1007/BF01953051. [DOI] [PubMed] [Google Scholar]
- Borea P. A., Bertelli G. M., Gilli G. Temperature dependence of the binding of mu, delta and kappa agonists to the opiate receptors in guinea-pig brain. Eur J Pharmacol. 1988 Feb 9;146(2-3):247–252. doi: 10.1016/0014-2999(88)90299-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson L. L., Weaver D. R., Reppert S. M. Melatonin signal transduction in hamster brain: inhibition of adenylyl cyclase by a pertussis toxin-sensitive G protein. Endocrinology. 1989 Nov;125(5):2670–2676. doi: 10.1210/endo-125-5-2670. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chong N. W., Sugden D. Guanine nucleotides regulate 2-[125I]iodomelatonin binding sites in chick retinal pigment epithelium but not in neuronal retina. J Neurochem. 1991 Aug;57(2):685–689. doi: 10.1111/j.1471-4159.1991.tb03800.x. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L. Characterization of a retinal melatonin receptor. J Pharmacol Exp Ther. 1985 Aug;234(2):395–401. [PubMed] [Google Scholar]
- Dubocovich M. L. Luzindole (N-0774): a novel melatonin receptor antagonist. J Pharmacol Exp Ther. 1988 Sep;246(3):902–910. [PubMed] [Google Scholar]
- Dubocovich M. L. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983 Dec 22;306(5945):782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Edelhoch H., Osborne J. C., Jr The thermodynamic basis of the stability of proteins, nucleic acids, and membranes. Adv Protein Chem. 1976;30:183–250. doi: 10.1016/s0065-3233(08)60480-5. [DOI] [PubMed] [Google Scholar]
- Gies J. P., Ilien B., Landry Y. Muscarinic acetylcholine receptor: thermodynamic analysis of the interaction of agonists and antagonists. Biochim Biophys Acta. 1986 Oct 31;889(1):103–115. doi: 10.1016/0167-4889(86)90014-5. [DOI] [PubMed] [Google Scholar]
- Kilpatrick G. J., el Tayar N., Van de Waterbeemd H., Jenner P., Testa B., Marsden C. D. The thermodynamics of agonist and antagonist binding to dopamine D-2 receptors. Mol Pharmacol. 1986 Sep;30(3):226–234. [PubMed] [Google Scholar]
- Krause D. N., Dubocovich M. L. Melatonin receptors. Annu Rev Pharmacol Toxicol. 1991;31:549–568. doi: 10.1146/annurev.pa.31.040191.003001. [DOI] [PubMed] [Google Scholar]
- Mei L., Wang J. X., Roeske W. R., Yamamura H. I. Thermodynamic analyses of pirenzepine binding to membrane-bound and solubilized muscarinic receptors from rat forebrain and heart. J Pharmacol Exp Ther. 1987 Sep;242(3):991–1000. [PubMed] [Google Scholar]
- Miklavc A., Kocjan D., Mavri J., Koller J., Hadzi D. On the fundamental difference in the thermodynamics of agonist and antagonist interactions with beta-adrenergic receptors and the mechanism of entropy-driven binding. Biochem Pharmacol. 1990 Aug 15;40(4):663–669. doi: 10.1016/0006-2952(90)90299-z. [DOI] [PubMed] [Google Scholar]
- Morgan P. J., Williams L. M. Central melatonin receptors: implications for a mode of action. Experientia. 1989 Oct 15;45(10):955–965. doi: 10.1007/BF01953053. [DOI] [PubMed] [Google Scholar]
- Möhler H., Richards J. G. Agonist and antagonist benzodiazepine receptor interaction in vitro. Nature. 1981 Dec 24;294(5843):763–765. doi: 10.1038/294763a0. [DOI] [PubMed] [Google Scholar]
- Paterson A., Chong N. W., Brinklow B. R., Loudon A. S., Sugden D. Characterization of 2-[125I]iodomelatonin binding sites in the brain of a marsupial, Bennett's wallaby (Macropus rufogriseus rufogriseus). Comp Biochem Physiol Comp Physiol. 1992 May;102(1):55–58. doi: 10.1016/0300-9629(92)90011-e. [DOI] [PubMed] [Google Scholar]
- Raffa R. B., Porreca F. Thermodynamic analysis of the drug-receptor interaction. Life Sci. 1989;44(4):245–258. doi: 10.1016/0024-3205(89)90182-3. [DOI] [PubMed] [Google Scholar]
- Rivkees S. A., Carlson L. L., Reppert S. M. Guanine nucleotide-binding protein regulation of melatonin receptors in lizard brain. Proc Natl Acad Sci U S A. 1989 May;86(10):3882–3886. doi: 10.1073/pnas.86.10.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. D., Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry. 1981 May 26;20(11):3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Speth R. C., Wastek G. J., Yamamura H. I. Benzodiazepine receptors: temperature dependence of [3H]flunitrazepam binding. Life Sci. 1979 Jan 22;24(4):351–357. doi: 10.1016/0024-3205(79)90331-x. [DOI] [PubMed] [Google Scholar]
- Sugden D. Aggregation of pigment granules in single cultured Xenopus laevis melanophores by melatonin analogues. Br J Pharmacol. 1991 Dec;104(4):922–927. doi: 10.1111/j.1476-5381.1991.tb12527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden D., Chong N. W. Pharmacological identity of 2-[125I]iodomelatonin binding sites in chicken brain and sheep pars tuberalis. Brain Res. 1991 Jan 18;539(1):151–154. doi: 10.1016/0006-8993(91)90698-u. [DOI] [PubMed] [Google Scholar]
- Testa B., Jenner P., Kilpatrick G. J., el Tayar N., Van de Waterbeemd H., Marsden C. D. Do thermodynamic studies provide information on both the binding to and the activation of dopaminergic and other receptors? Biochem Pharmacol. 1987 Dec 1;36(23):4041–4046. doi: 10.1016/0006-2952(87)90559-4. [DOI] [PubMed] [Google Scholar]
- Vanecek J., Klein D. C. Melatonin inhibits gonadotropin-releasing hormone-induced elevation of intracellular Ca2+ in neonatal rat pituitary cells. Endocrinology. 1992 Feb;130(2):701–707. doi: 10.1210/endo.130.2.1733718. [DOI] [PubMed] [Google Scholar]
- Vanecek J., Vollrath L. Melatonin inhibits cyclic AMP and cyclic GMP accumulation in the rat pituitary. Brain Res. 1989 Dec 25;505(1):157–159. doi: 10.1016/0006-8993(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M., Camus J., Tastenoy M., Lambrecht G., Mutschler E., Kropfgans M., Sperlich J., Wiesenberger F., Tacke R., Christophe J. Thermodynamics of antagonist binding to rat muscarinic M2 receptors: antimuscarinics of the pridinol, sila-pridinol, diphenidol and sila-diphenidol type. Br J Pharmacol. 1993 Jun;109(2):360–370. doi: 10.1111/j.1476-5381.1993.tb13578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelbroeck M., Robberecht P., Chatelain P., De Neef P., Christophe J. Effects of temperature and ethanol on agonist and antagonist binding to rat heart muscarinic receptors in the absence and presence of GTP. Biochem J. 1985 Oct 15;231(2):469–476. doi: 10.1042/bj2310469. [DOI] [PMC free article] [PubMed] [Google Scholar]



