Abstract
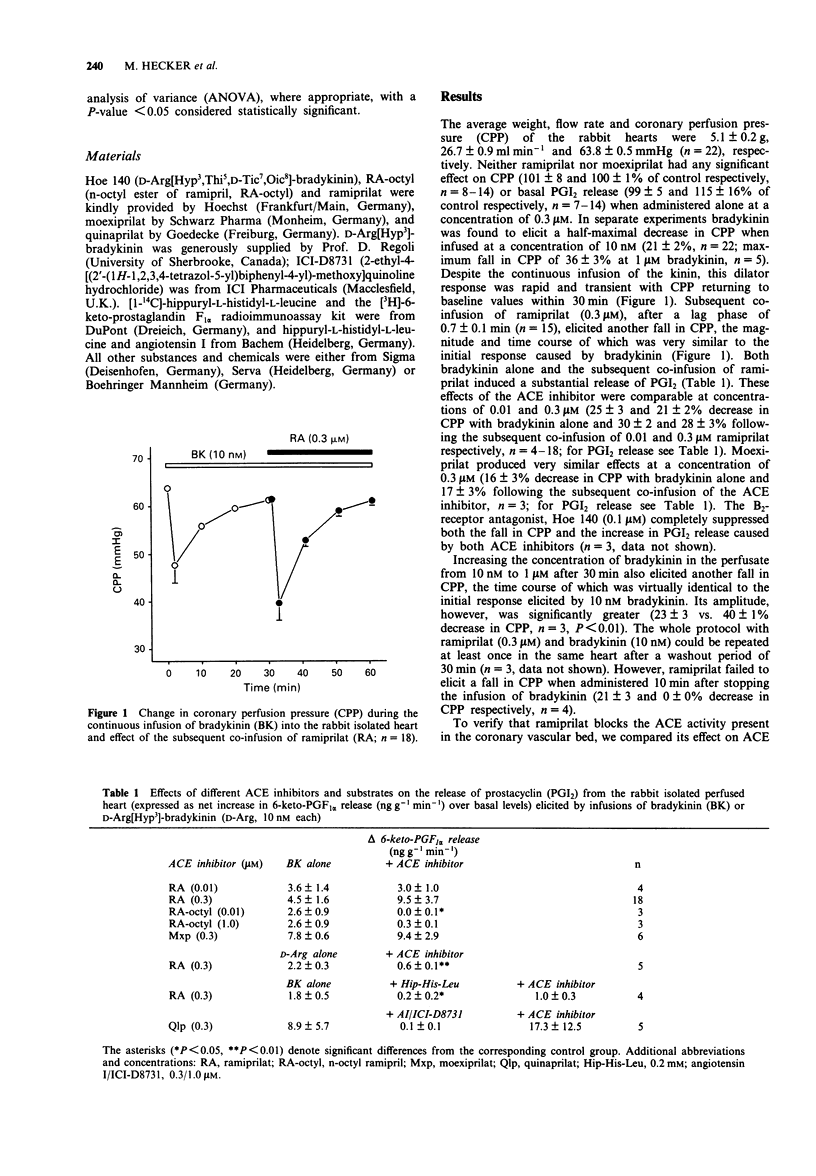
1. To examine the possibility that angiotensin-converting enzyme (ACE) inhibitors modulate the action of bradykinin at the receptor level, their effect on the dilator response to bradykinin was studied in the isolated saline-perfused heart of the rabbit. 2. Continuous infusion of bradykinin (10 nM) elicited a transient decrease in coronary perfusion pressure (CPP) and increased prostacyclin (PGI2) release which returned to baseline values within 30 min. 3. Subsequent co-infusion of ramiprilat (> or = 10 nM) or moexiprilat, but not of the less potent ACE inhibitor n-octyl-ramipril (RA-octyl), caused another fall in CPP and an increase in PGI2 release, the magnitude and time course of which were almost identical to the first response to bradykinin. No change in CPP or PGI2 release was observed when the ACE inhibitors were administered in the absence of exogenous bradykinin. 4. Infusion of D-Arg[Hyp3]-bradykinin (10 nM), a specific B2-receptor agonist which was significantly more resistant to degradation by ACE than bradykinin, produced virtually identical changes in CPP and PGI2 release when compared to bradykinin. Subsequent co-infusion of ramiprilat was similarly effective in restoring the fall in CPP and increase in PGI2 release elicited by D-Arg[Hyp3]-bradykinin as in the presence of bradykinin. 5. In concentrations which should block the degradation of bradykinin by ACE in the coronary vascular bed, two ACE substrates, hippuryl-L-histidyl-L-leucine (0.2 mM) and angiotensin I (0.3 microM), were unable to elicit a significant change in CPP or PGI2 release while ramiprilat and another ACE inhibitor, quinaprilat, were still active in the presence of these substrates.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

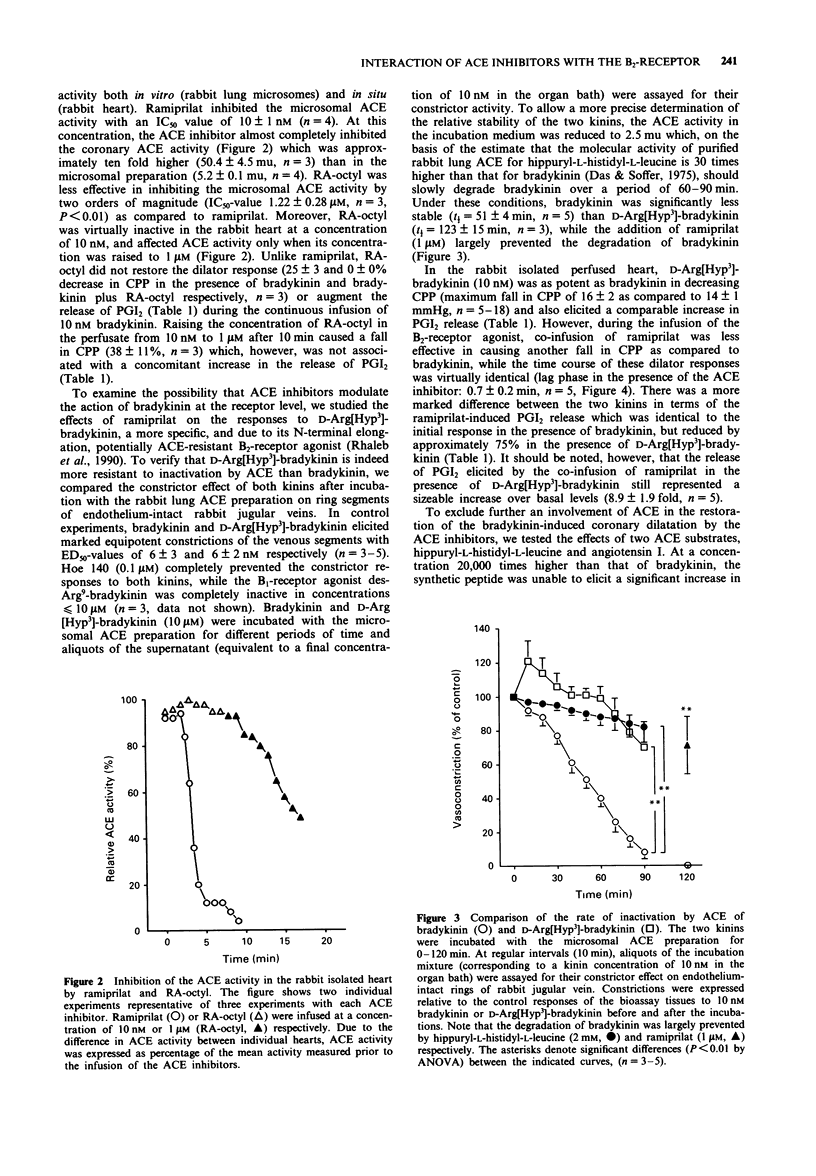
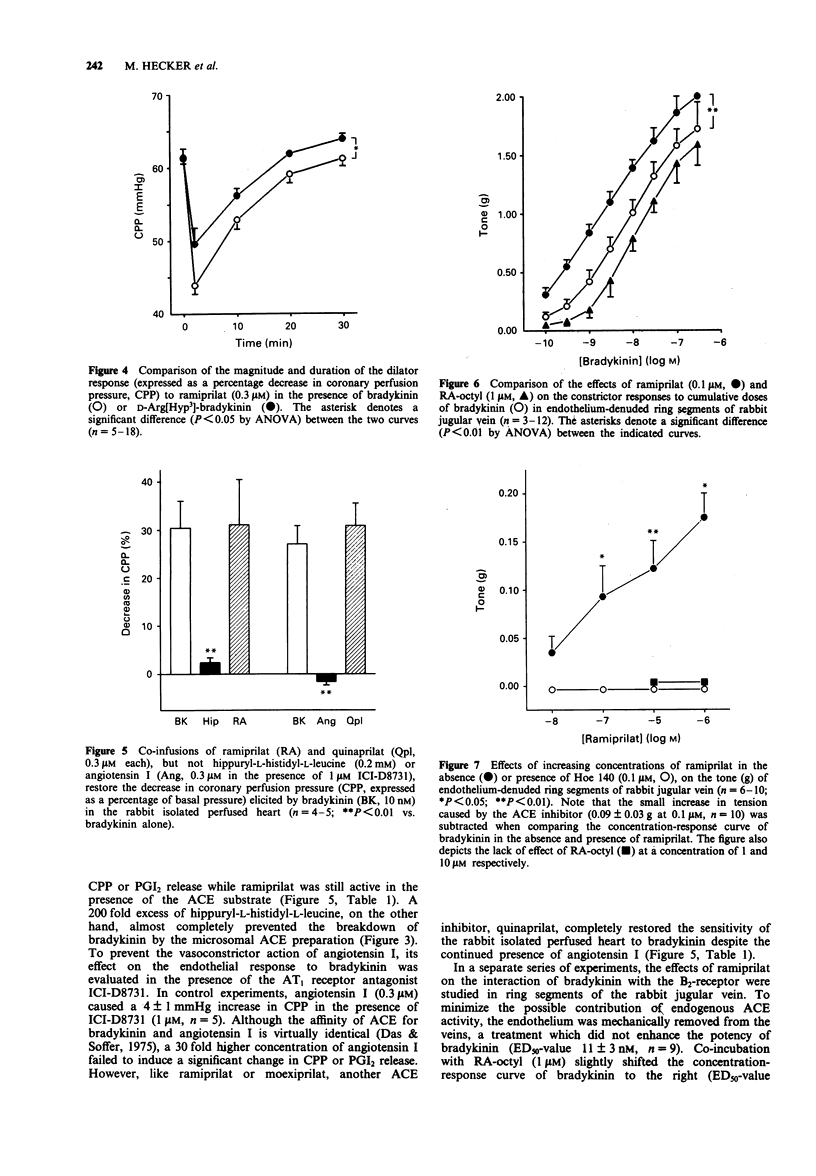


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auch-Schwelk W., Bossaller C., Claus M., Graf K., Gräfe M., Fleck E. ACE inhibitors are endothelium dependent vasodilators of coronary arteries during submaximal stimulation with bradykinin. Cardiovasc Res. 1993 Feb;27(2):312–317. doi: 10.1093/cvr/27.2.312. [DOI] [PubMed] [Google Scholar]
- Bathon J. M., Proud D. Bradykinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:129–162. doi: 10.1146/annurev.pa.31.040191.001021. [DOI] [PubMed] [Google Scholar]
- Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992 Mar;44(1):1–80. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Busse R., Fleming I., Hecker M. Endothelium-derived bradykinin: implications for angiotensin-converting enzyme-inhibitor therapy. J Cardiovasc Pharmacol. 1993;22 (Suppl 5):S31–S36. [PubMed] [Google Scholar]
- Busse R., Lamontagne D. Endothelium-derived bradykinin is responsible for the increase in calcium produced by angiotensin-converting enzyme inhibitors in human endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 1991 Jul;344(1):126–129. doi: 10.1007/BF00167392. [DOI] [PubMed] [Google Scholar]
- Das M., Soffer R. L. Pulmonary angiotensin-converting enzyme. Structural and catalytic properties. J Biol Chem. 1975 Sep 10;250(17):6762–6768. [PubMed] [Google Scholar]
- Erdös E. G. Some old and some new ideas on kinin metabolism. J Cardiovasc Pharmacol. 1990;15 (Suppl 6):S20–S24. [PubMed] [Google Scholar]
- Etscheid B. G., Villereal M. L. Coupling of bradykinin receptors to phospholipase C in cultured fibroblasts is mediated by a G-protein. J Cell Physiol. 1989 Aug;140(2):264–271. doi: 10.1002/jcp.1041400211. [DOI] [PubMed] [Google Scholar]
- Hecker M., Bara A. T., Busse R. Relaxation of isolated coronary arteries by angiotensin-converting enzyme inhibitors: role of endothelium-derived kinins. J Vasc Res. 1993 Sep-Oct;30(5):257–262. doi: 10.1159/000159004. [DOI] [PubMed] [Google Scholar]
- Hecker M., Dambacher T., Busse R. Role of endothelium-derived bradykinin in the control of vascular tone. J Cardiovasc Pharmacol. 1992;20 (Suppl 9):S55–S61. [PubMed] [Google Scholar]
- Iimura O., Shimamoto K. Role of kallikrein-kinin system in the hypotensive mechanisms of converting enzyme inhibitors in essential hypertension. J Cardiovasc Pharmacol. 1989;13 (Suppl 3):S63–S66. doi: 10.1097/00005344-198900133-00016. [DOI] [PubMed] [Google Scholar]
- Kiowski W., Linder L., Kleinbloesem C., van Brummelen P., Bühler F. R. Blood pressure control by the renin-angiotensin system in normotensive subjects. Assessment by angiotensin converting enzyme and renin inhibition. Circulation. 1992 Jan;85(1):1–8. doi: 10.1161/01.cir.85.1.1. [DOI] [PubMed] [Google Scholar]
- Lamontagne D., König A., Bassenge E., Busse R. Prostacyclin and nitric oxide contribute to the vasodilator action of acetylcholine and bradykinin in the intact rabbit coronary bed. J Cardiovasc Pharmacol. 1992 Oct;20(4):652–657. doi: 10.1097/00005344-199210000-00020. [DOI] [PubMed] [Google Scholar]
- Mombouli J. V., Illiano S., Nagao T., Scott-Burden T., Vanhoutte P. M. Potentiation of endothelium-dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium-derived relaxing and hyperpolarizing factors. Circ Res. 1992 Jul;71(1):137–144. doi: 10.1161/01.res.71.1.137. [DOI] [PubMed] [Google Scholar]
- Munoz C. M., Leeb-Lundberg L. M. Receptor-mediated internalization of bradykinin. DDT1 MF-2 smooth muscle cells process internalized bradykinin via multiple degradative pathways. J Biol Chem. 1992 Jan 5;267(1):303–309. [PubMed] [Google Scholar]
- Newby A. C., Henderson A. H. Stimulus-secretion coupling in vascular endothelial cells. Annu Rev Physiol. 1990;52:661–674. doi: 10.1146/annurev.ph.52.030190.003305. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Rhaleb N. E., Drapeau G., Dion S., Jukic D., Rouissi N., Regoli D. Structure-activity studies on bradykinin and related peptides: agonists. Br J Pharmacol. 1990 Mar;99(3):445–448. doi: 10.1111/j.1476-5381.1990.tb12947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscher A. A., Manganiello V. C., Jelsema C. L., Moss J. Autoregulation of bradykinin receptors and bradykinin-induced prostacyclin formation in human fibroblasts. J Clin Invest. 1984 Aug;74(2):552–558. doi: 10.1172/JCI111452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schini V. B., Boulanger C., Regoli D., Vanhoutte P. M. Bradykinin stimulates the production of cyclic GMP via activation of B2 kinin receptors in cultured porcine aortic endothelial cells. J Pharmacol Exp Ther. 1990 Feb;252(2):581–585. [PubMed] [Google Scholar]
- Schmaier A. H., Kuo A., Lundberg D., Murray S., Cines D. B. The expression of high molecular weight kininogen on human umbilical vein endothelial cells. J Biol Chem. 1988 Nov 5;263(31):16327–16333. [PubMed] [Google Scholar]
- Weintraub W. H., Negulescu P. A., Machen T. E. Calcium signaling in endothelia: cellular heterogeneity and receptor internalization. Am J Physiol. 1992 Nov;263(5 Pt 1):C1029–C1039. doi: 10.1152/ajpcell.1992.263.5.C1029. [DOI] [PubMed] [Google Scholar]
- Wiemer G., Schölkens B. A., Becker R. H., Busse R. Ramiprilat enhances endothelial autacoid formation by inhibiting breakdown of endothelium-derived bradykinin. Hypertension. 1991 Oct;18(4):558–563. doi: 10.1161/01.hyp.18.4.558. [DOI] [PubMed] [Google Scholar]


