Abstract
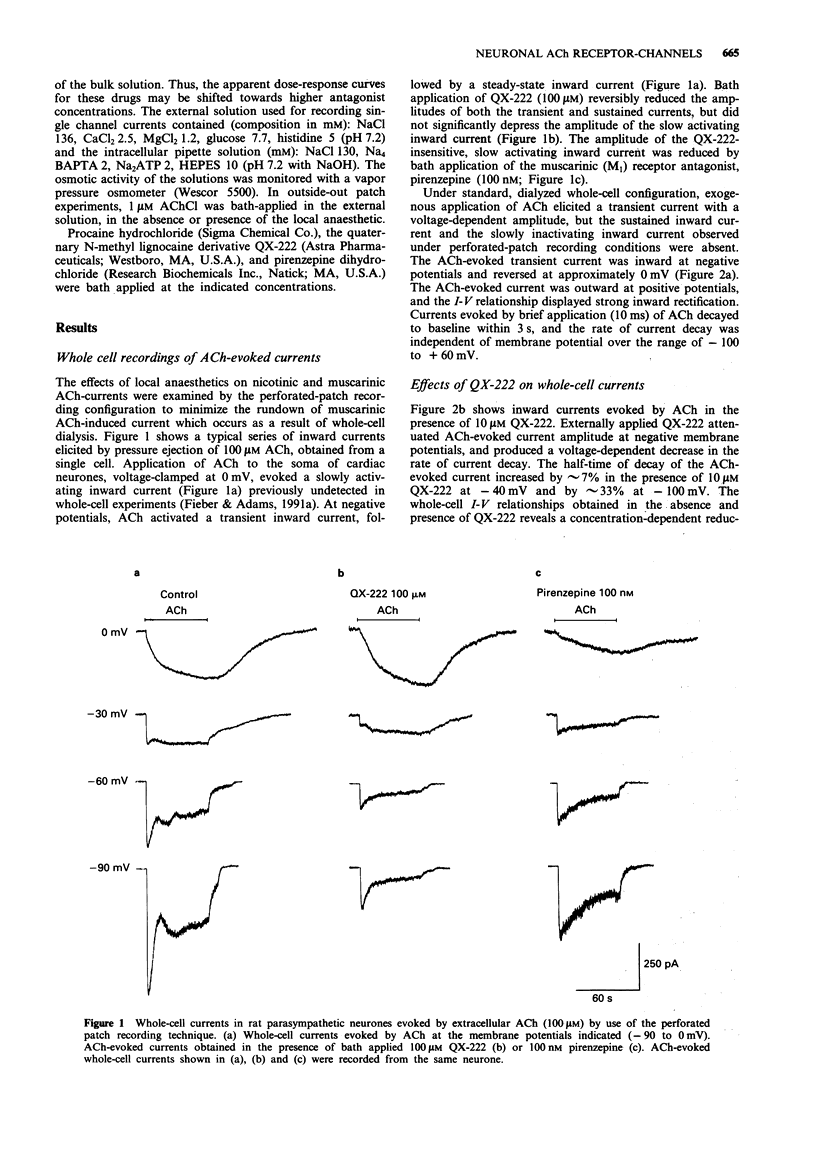
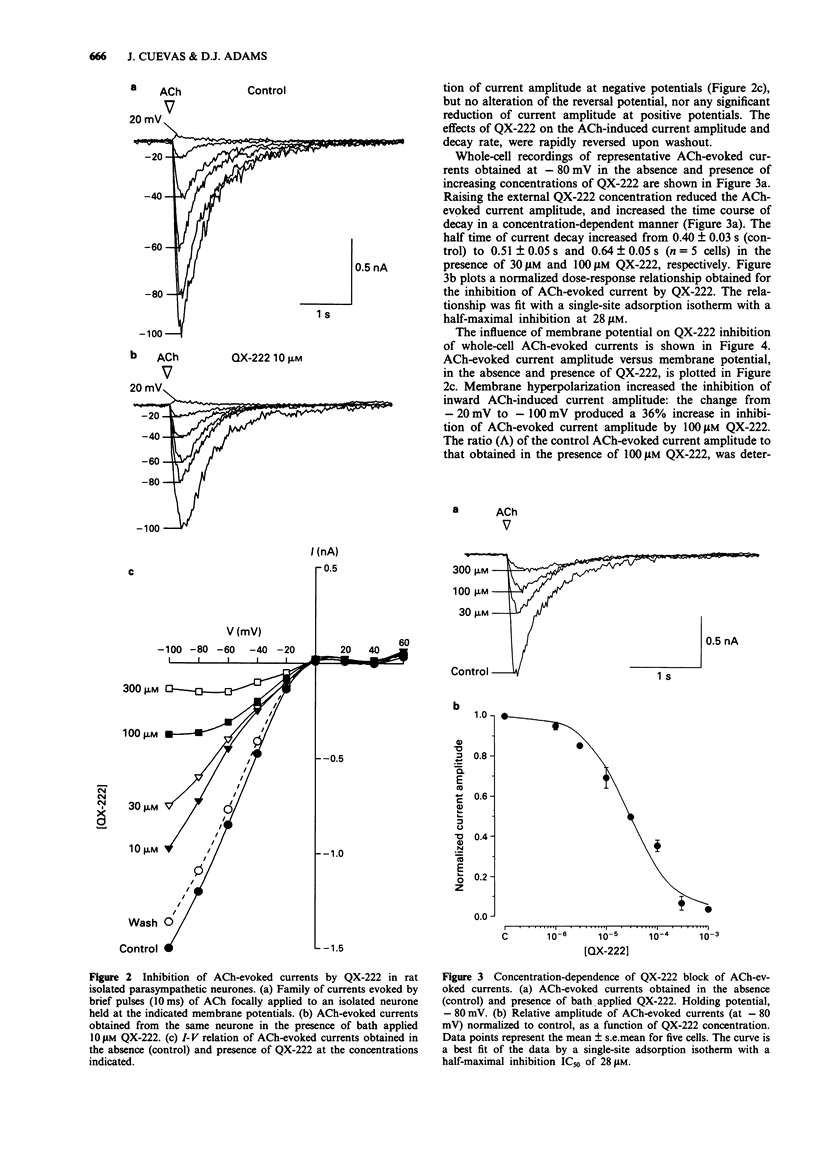
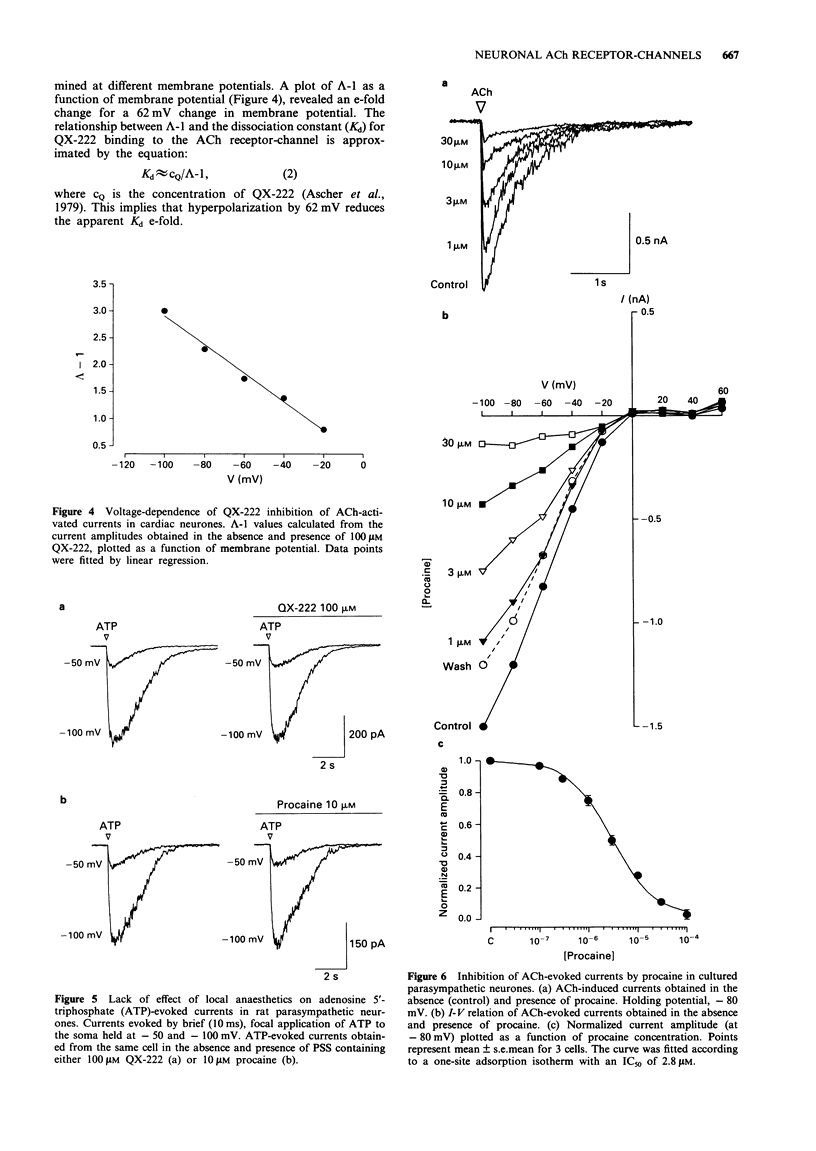
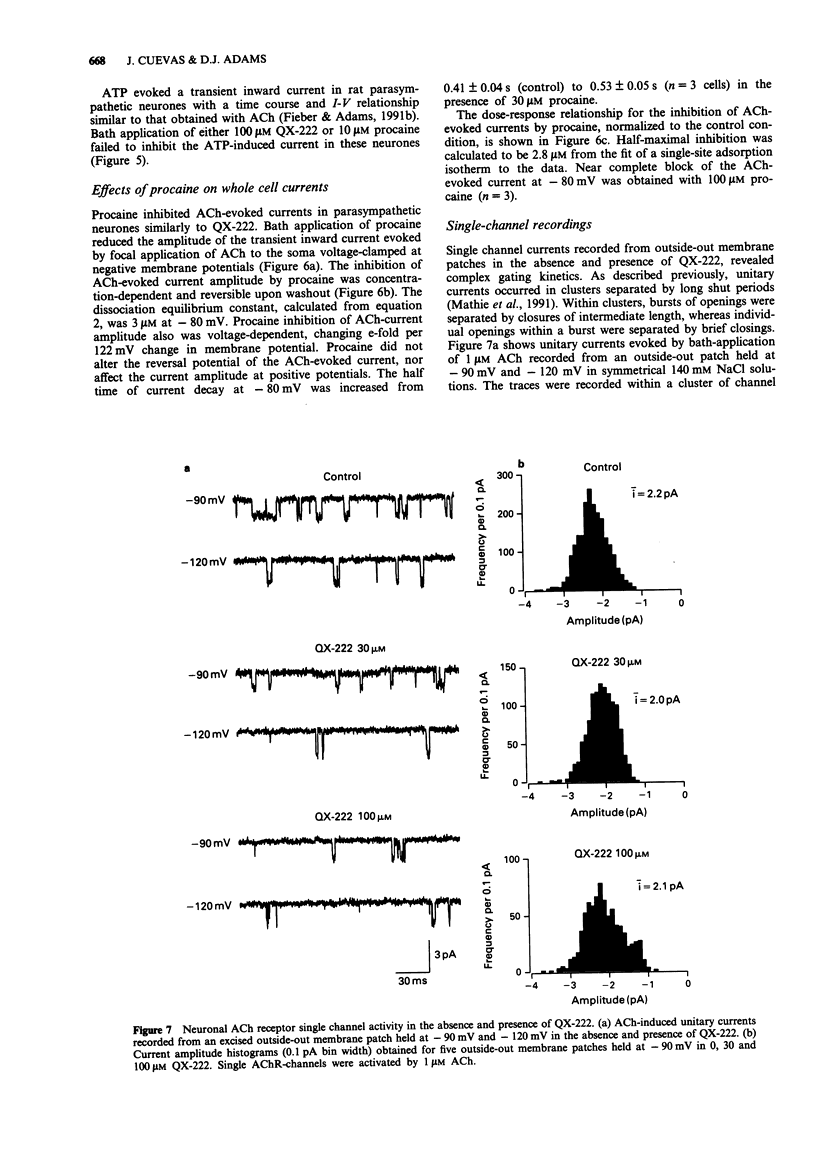
1 The effects of the local anaesthetics QX-222 and procaine on nicotinic acetylcholine (ACh)-evoked currents in cultured parasympathetic cardiac neurones of the rat were investigated by use of the whole-cell, perforated-patch, and outside-out recording configurations of the patch clamp method. 2 QX-222 and procaine, applied to the extracellular surface, reversibly inhibited the peak amplitude of the whole-cell nicotinic ACh-evoked current in a concentration-dependent manner, with half-maximal inhibitory concentrations (IC50) of 28 microM and 2.8 microM, respectively, at -80 mV. In these neurones, the sustained inward current mediated by M1 muscarinic receptor activation was unaltered by QX-222, and neither local anaesthetic affected the adenosine 5'-triphosphate (ATP)-evoked current. 3 QX-222 and procaine block of nicotinic ACh-evoked inward current was voltage-dependent and enhanced by hyperpolarization. An e-fold change in their dissociation equilibrium constants (Kd) resulted from a 62 mV and a 122 mV change in membrane potential, respectively. 4 Both local anaesthetics produce a concentration-dependent increase in the half-time of decay of the nicotinic ACh-evoked inward current. 5 Measurements of unitary currents in outside-out patches showed that QX-222 reversibly increased the mean burst duration and closed time and reduced the mean channel open time and open-state probability of the nicotinic ACh receptor-channel (AChR) in a concentration-dependent manner. 6 The Kd and voltage sensitivity of local anaesthetic block of the nicotinic AChR in rat intracardiac neurones suggests that the pore-forming region of this channel differs from that of the AChR in frog and rat skeletal muscle and from the neuronal alpha 4 beta 2 ACh receptor-channel.
Full text
PDF



Selected References
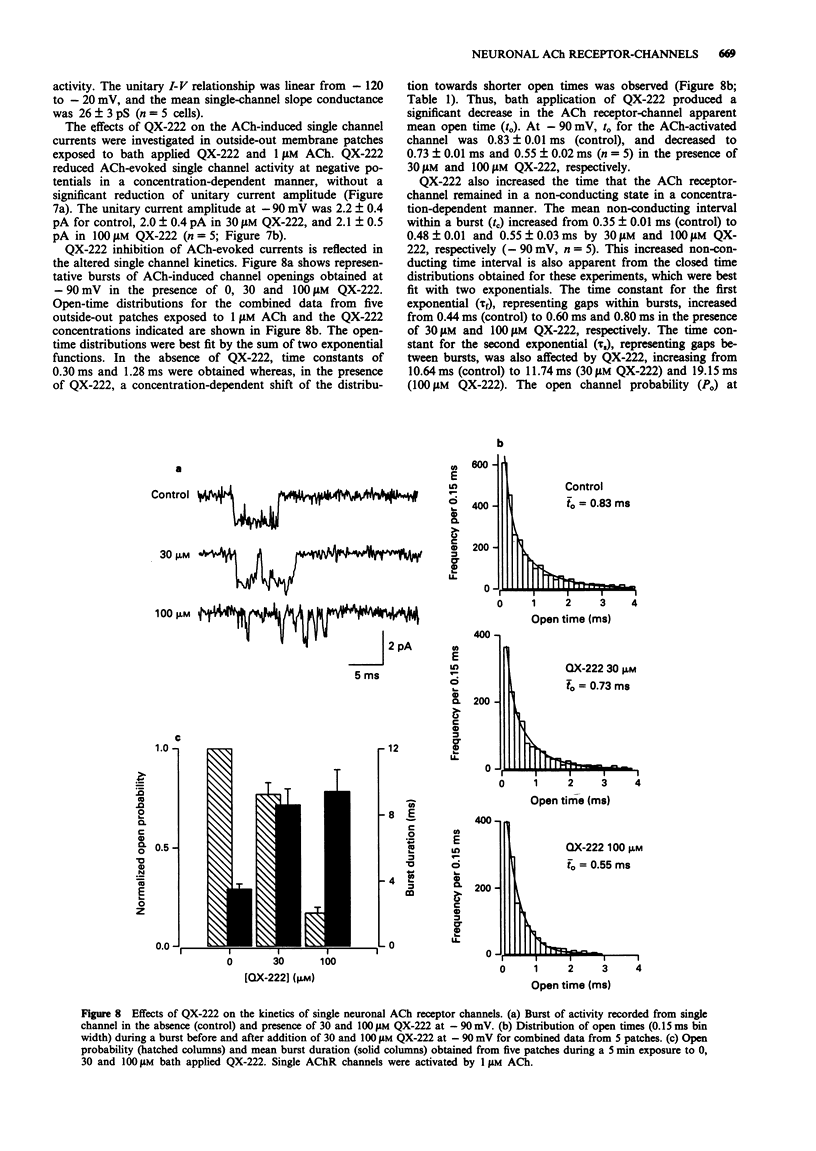
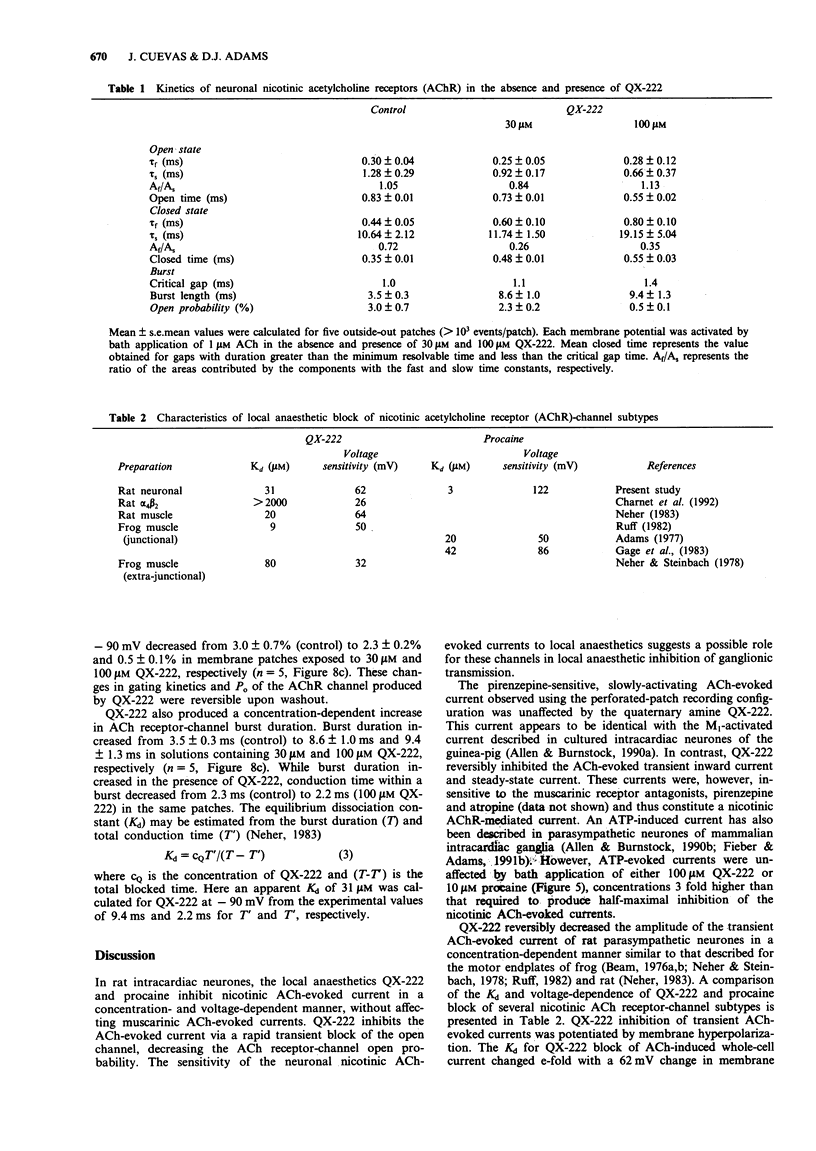
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Nutter T. J. Calcium permeability and modulation of nicotinic acetylcholine receptor-channels in rat parasympathetic neurons. J Physiol Paris. 1992;86(1-3):67–76. doi: 10.1016/s0928-4257(05)80009-9. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alary J. G., Brodeur J. Studies on the mechanism of phenobarbital-induced protection against parathion in adult female rats. J Pharmacol Exp Ther. 1969 Oct;169(2):159–167. [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. M1 and M2 muscarinic receptors mediate excitation and inhibition of guinea-pig intracardiac neurones in culture. J Physiol. 1990 Mar;422:463–480. doi: 10.1113/jphysiol.1990.sp017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. The actions of adenosine 5'-triphosphate on guinea-pig intracardiac neurones in culture. Br J Pharmacol. 1990 Jun;100(2):269–276. doi: 10.1111/j.1476-5381.1990.tb15794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Conroy W. G., Schoepfer R., Whiting P., Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem. 1991 Jun 15;266(17):11192–11198. [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A quantitative description of end-plate currents in the presence of two lidocaine derivatives. J Physiol. 1976 Jun;258(2):301–322. doi: 10.1113/jphysiol.1976.sp011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth P., Jacobson I., Pocock G., Richards C. D. The mechanism by which procaine inhibits catecholamine secretion from bovine chromaffin cells. Br J Pharmacol. 1992 Aug;106(4):802–812. doi: 10.1111/j.1476-5381.1992.tb14416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Cohen B. N., Davidson N., Lester H. A., Pilar G. Pharmacological and kinetic properties of alpha 4 beta 2 neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Physiol. 1992 May;450:375–394. doi: 10.1113/jphysiol.1992.sp019132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Couturier S., Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991 Mar 21;350(6315):235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Dani J. A. Site-directed mutagenesis and single-channel currents define the ionic channel of the nicotinic acetylcholine receptor. Trends Neurosci. 1989 Apr;12(4):125–128. doi: 10.1016/0166-2236(89)90049-0. [DOI] [PubMed] [Google Scholar]
- Deneris E. S., Connolly J., Rogers S. W., Duvoisin R. Pharmacological and functional diversity of neuronal nicotinic acetylcholine receptors. Trends Pharmacol Sci. 1991 Jan;12(1):34–40. doi: 10.1016/0165-6147(91)90486-c. [DOI] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Acetylcholine-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hamill O. P., Wachtel R. E. Sites of action of procaine at the motor end-plate. J Physiol. 1983 Feb;335:123–137. doi: 10.1113/jphysiol.1983.sp014524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hassall C. J., Buckley N. J., Burnstock G. Autoradiographic localisation of muscarinic receptors on guinea pig intracardiac neurones and atrial myocytes in culture. Neurosci Lett. 1987 Feb 24;74(2):145–150. doi: 10.1016/0304-3940(87)90140-6. [DOI] [PubMed] [Google Scholar]
- Horn R., Brodwick M. S., Dickey W. D. Asymmetry of the acetylcholine channel revealed by quaternary anesthetics. Science. 1980 Oct 10;210(4466):205–207. doi: 10.1126/science.6251552. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988 Dec 16;242(4885):1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Luetje C. W., Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991 Mar;11(3):837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje C. W., Wada K., Rogers S., Abramson S. N., Tsuji K., Heinemann S., Patrick J. Neurotoxins distinguish between different neuronal nicotinic acetylcholine receptor subunit combinations. J Neurochem. 1990 Aug;55(2):632–640. doi: 10.1111/j.1471-4159.1990.tb04180.x. [DOI] [PubMed] [Google Scholar]
- Lukas R. J., Bencherif M. Heterogeneity and regulation of nicotinic acetylcholine receptors. Int Rev Neurobiol. 1992;34:25–131. doi: 10.1016/s0074-7742(08)60097-5. [DOI] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Conductance and kinetic properties of single nicotinic acetylcholine receptor channels in rat sympathetic neurones. J Physiol. 1991 Aug;439:717–750. doi: 10.1113/jphysiol.1991.sp018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R. L. The kinetic properties of neuronal nicotinic receptors: genetic basis of functional diversity. Prog Neurobiol. 1993 Oct;41(4):509–531. doi: 10.1016/0301-0082(93)90028-q. [DOI] [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. The kinetics of local anesthetic blockade of end-plate channels. Biophys J. 1982 Mar;37(3):625–631. [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Fieber L. A., Adams D. J. Neurotransmission in neonatal rat cardiac ganglion in situ. Am J Physiol. 1990 Oct;259(4 Pt 2):H997–1005. doi: 10.1152/ajpheart.1990.259.4.H997. [DOI] [PubMed] [Google Scholar]
- Selyanko A. A., Skok V. I. Acetylcholine receptors in rat cardiac neurones. J Auton Nerv Syst. 1992 Aug;40(1):33–47. doi: 10.1016/0165-1838(92)90223-4. [DOI] [PubMed] [Google Scholar]
- Tabatabai M., Booth A. M. Mechanism of action of local anesthetics on synaptic transmission in the rat. Anesth Analg. 1990 Aug;71(2):149–157. doi: 10.1213/00000539-199008000-00007. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


