Abstract
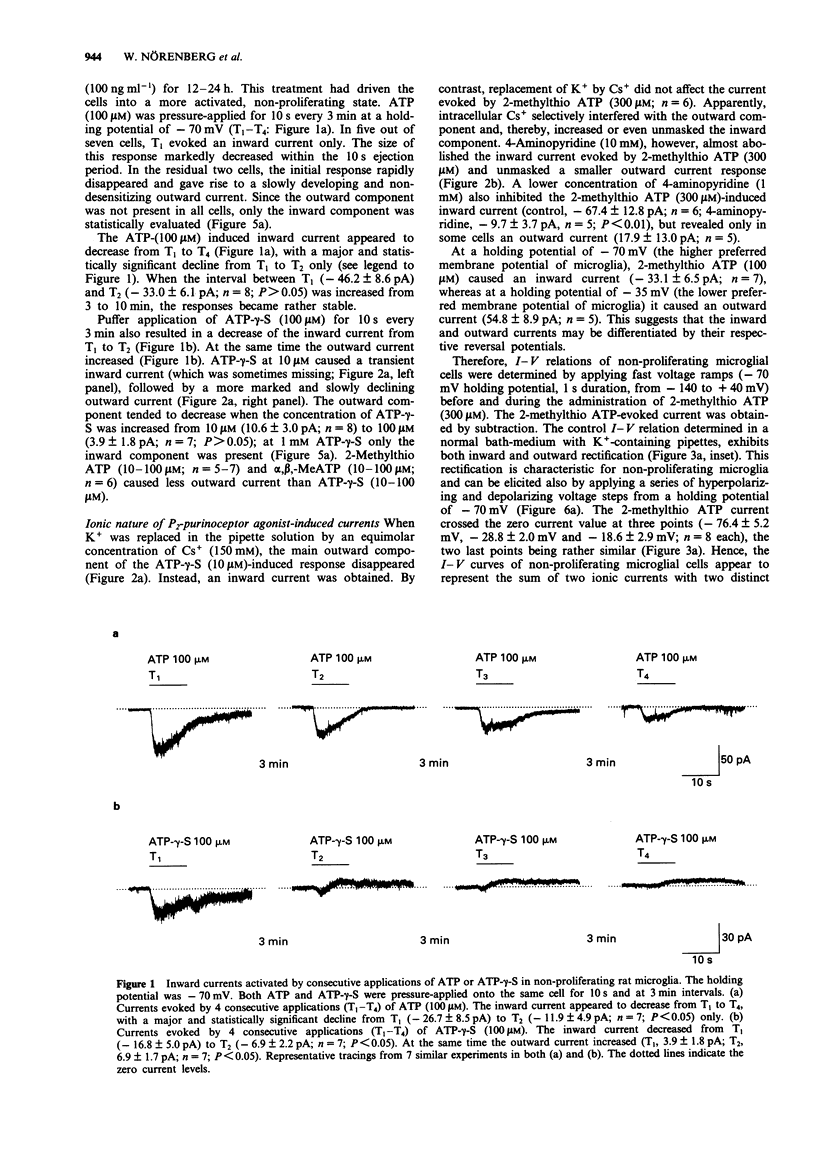
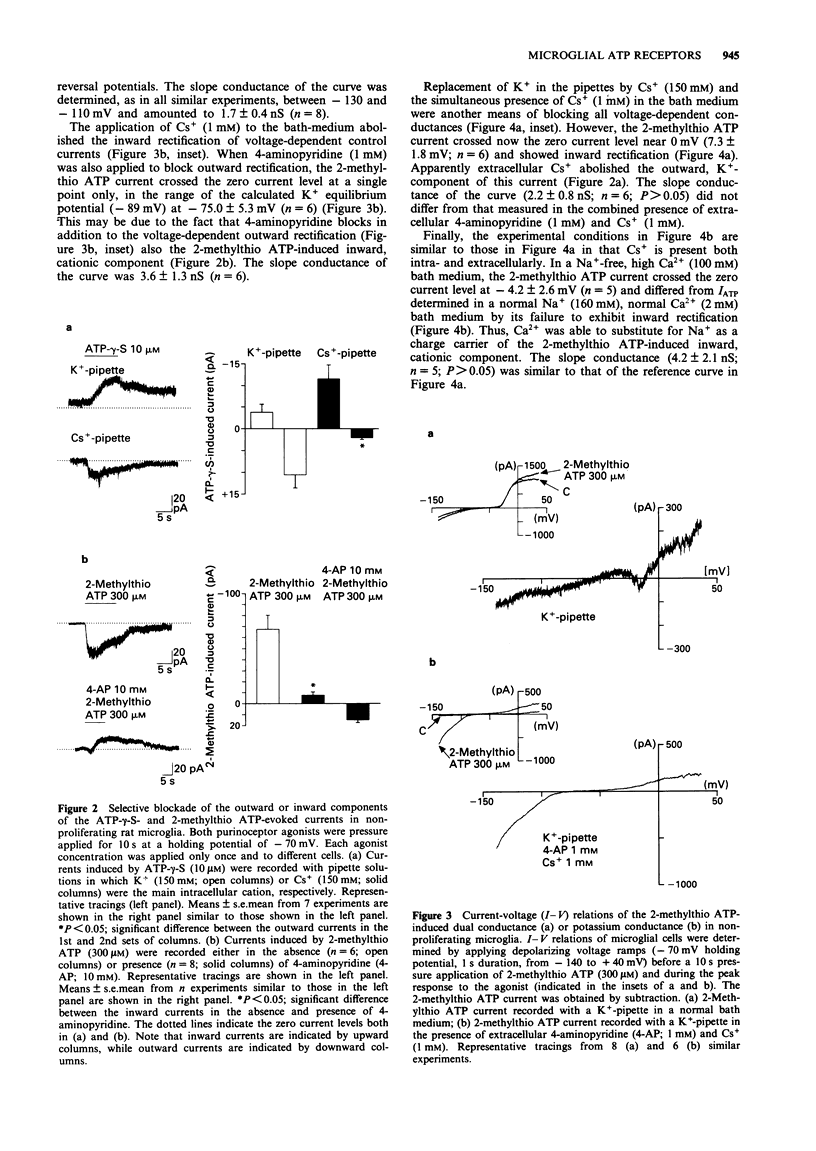
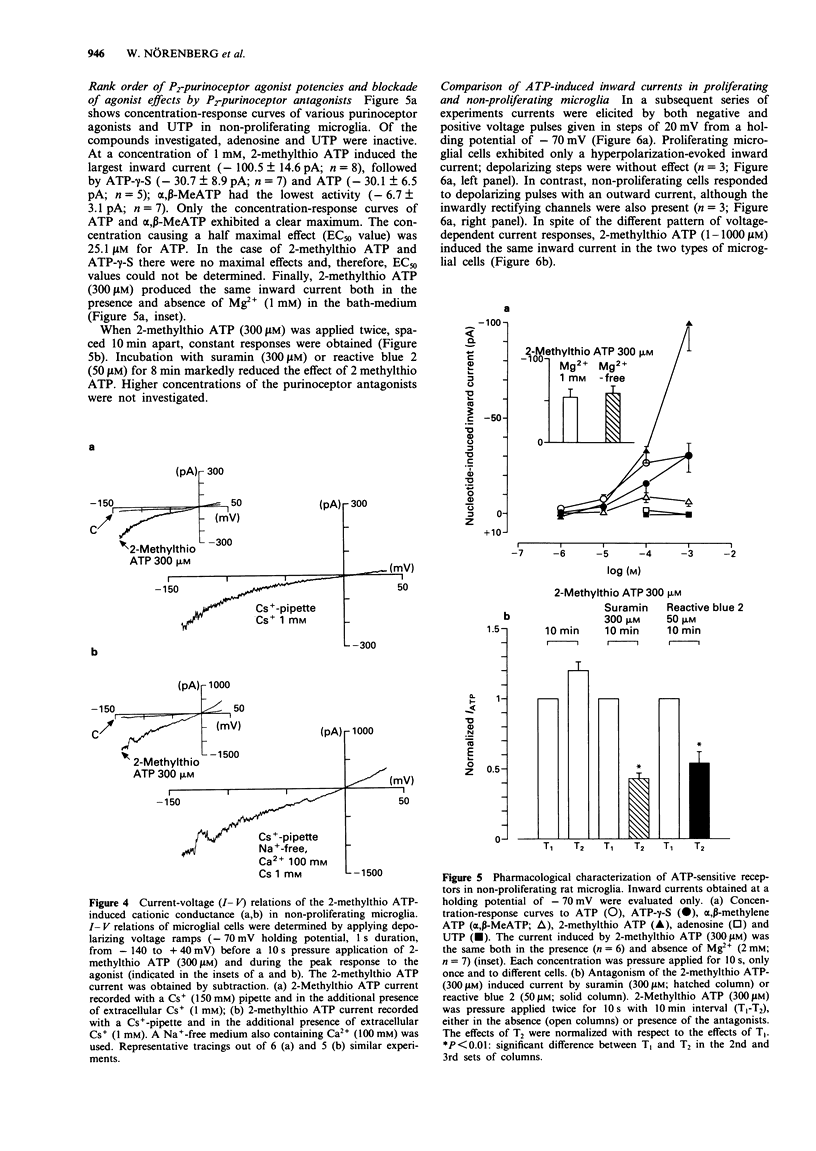
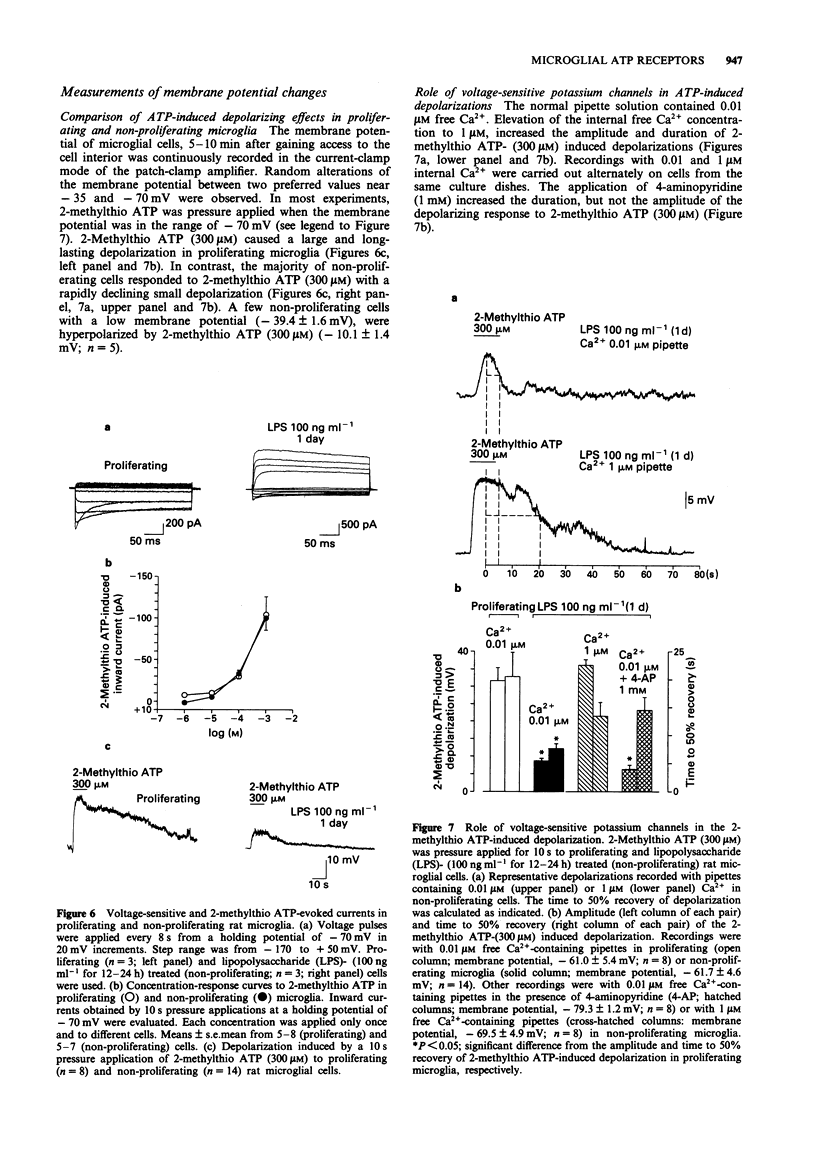
1. Purinoceptor agonist-induced currents in untreated (proliferating) and lipopolysaccharide (LPS; 100 ng ml-1)-treated (non-proliferating) rat microglial cells in culture were recorded by the whole-cell patch-clamp technique. These cells have two preferred resting membrane potentials, one at -35 mV and another one at -70 mV. 2. Most experiments were carried out in non-proliferating cells. ATP, ATP-gamma-S and alpha,beta-MeATP (1-1000 microM in all cases) evoked an inward current at a holding potential of -70 mV, followed, in some experiments, by an outward current. At -70 mV 2-methylthio ATP (1-1000 microM) evoked an inward current, whereas at -35 mV it produced an outward current only. 3. When K+ was replaced in the pipette solution by an equimolar concentration of Cs+ (150 mM), the main outward component of the ATP-gamma-S (10 microM) induced response disappeared. Instead, an inward current was obtained. Replacement of K+ by Cs+ did not affect the inward current evoked by 2-methylthio ATP (300 microM). 4-Aminopyridine (1-10 mM), however, almost abolished this current and unmasked a smaller outward current. 4. The rank order of agonist potency was 2-methylthio ATP > ATP > alpha,beta-MeATP. Adenosine and UTP were inactive. Suramin (300 microM) and reactive blue 2 (50 microM) antagonized the effect of 2-methylthio ATP (300 microM). 5. I-V relations were determined by delivering fast voltage ramps before and during the application of 2-methylthio ATP (300 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. Molecular transductional mechanisms by which IFN gamma and other signals regulate macrophage development. Immunol Rev. 1987 Jun;97:5–27. doi: 10.1111/j.1600-065x.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Banati R. B., Hoppe D., Gottmann K., Kreutzberg G. W., Kettenmann H. A subpopulation of bone marrow-derived macrophage-like cells shares a unique ion channel pattern with microglia. J Neurosci Res. 1991 Dec;30(4):593–600. doi: 10.1002/jnr.490300402. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Friel D. D. ATP-activated channels in excitable cells. Ion Channels. 1990;2:169–203. doi: 10.1007/978-1-4615-7305-0_5. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisman H. P., Steinberg T. H., Fischbarg J., Silverstein S. C., Vogelzang S. A., Ince C., Ypey D. L., Leijh P. C. Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7988–7992. doi: 10.1073/pnas.85.21.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Christie A., Sharma V. K., Sheu S. S. Mechanism of extracellular ATP-induced increase of cytosolic Ca2+ concentration in isolated rat ventricular myocytes. J Physiol. 1992 Jan;445:369–388. doi: 10.1113/jphysiol.1992.sp018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D. W., Mattiace L. A., Kure K., Hutchins K., Lyman W. D., Brosnan C. F. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991 Feb;64(2):135–156. [PubMed] [Google Scholar]
- Gallin E. K. Voltage clamp studies in macrophages from mouse spleen cultures. Science. 1981 Oct 23;214(4519):458–460. doi: 10.1126/science.7291986. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter P. J., Bauer J., Schobert A., Northoff H. Lipopolysaccharide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. J Neurosci. 1989 Jan;9(1):183–194. doi: 10.1523/JNEUROSCI.09-01-00183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hara N., Ichinose M., Sawada M., Imai K., Maeno T. Activation of single Ca2(+)-dependent K+ channel by external ATP in mouse macrophages. FEBS Lett. 1990 Jul 16;267(2):281–284. doi: 10.1016/0014-5793(90)80945-f. [DOI] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993 Feb;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Jordan F. L., Thomas W. E. Brain macrophages: questions of origin and interrelationship. Brain Res. 1988 Apr-Jun;472(2):165–178. doi: 10.1016/0165-0173(88)90019-7. [DOI] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Kettenmann H., Banati R., Walz W. Electrophysiological behavior of microglia. Glia. 1993 Jan;7(1):93–101. doi: 10.1002/glia.440070115. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Hoppe D., Gottmann K., Banati R., Kreutzberg G. Cultured microglial cells have a distinct pattern of membrane channels different from peritoneal macrophages. J Neurosci Res. 1990 Jul;26(3):278–287. doi: 10.1002/jnr.490260303. [DOI] [PubMed] [Google Scholar]
- Maltsev V. A. A negative resistance region underlies the triggering property of membrane potential in human T-lymphocytes. Cell Signal. 1992 Nov;4(6):697–707. doi: 10.1016/0898-6568(92)90050-i. [DOI] [PubMed] [Google Scholar]
- Naumov A. P., Kuryshev Y. A., Kaznacheyeva E. V., Mozhayeva G. N. ATP-activated Ca(2+)-permeable channels in rat peritoneal macrophages. FEBS Lett. 1992 Nov 30;313(3):285–287. doi: 10.1016/0014-5793(92)81210-d. [DOI] [PubMed] [Google Scholar]
- Nörenberg W., Appel K., Bauer J., Gebicke-Haerter P. J., Illes P. Expression of an outwardly rectifying K+ channel in rat microglia cultivated on teflon. Neurosci Lett. 1993 Sep 17;160(1):69–72. doi: 10.1016/0304-3940(93)9001-0. [DOI] [PubMed] [Google Scholar]
- Nörenberg W., Gebicke-Haerter P. J., Illes P. Inflammatory stimuli induce a new K+ outward current in cultured rat microglia. Neurosci Lett. 1992 Dec 7;147(2):171–174. doi: 10.1016/0304-3940(92)90587-w. [DOI] [PubMed] [Google Scholar]
- Rieske E., Graeber M. B., Tetzlaff W., Czlonkowska A., Streit W. J., Kreutzberg G. W. Microglia and microglia-derived brain macrophages in culture: generation from axotomized rat facial nuclei, identification and characterization in vitro. Brain Res. 1989 Jul 17;492(1-2):1–14. doi: 10.1016/0006-8993(89)90883-4. [DOI] [PubMed] [Google Scholar]
- Seifert R., Schultz G. Involvement of pyrimidinoceptors in the regulation of cell functions by uridine and by uracil nucleotides. Trends Pharmacol Sci. 1989 Sep;10(9):365–369. doi: 10.1016/0165-6147(89)90009-6. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987 Jun 25;262(18):8884–8888. [PubMed] [Google Scholar]
- Sung S. S., Young J. D., Origlio A. M., Heiple J. M., Kaback H. R., Silverstein S. C. Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+], and inhibits phagocytosis in mouse macrophages. J Biol Chem. 1985 Nov 5;260(25):13442–13449. [PubMed] [Google Scholar]
- Theele D. P., Streit W. J. A chronicle of microglial ontogeny. Glia. 1993 Jan;7(1):5–8. doi: 10.1002/glia.440070104. [DOI] [PubMed] [Google Scholar]
- Thomas W. E. Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992 Jan-Apr;17(1):61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Walz W., Ilschner S., Ohlemeyer C., Banati R., Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci. 1993 Oct;13(10):4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kügelgen I., Häussinger D., Starke K. Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):556–560. doi: 10.1007/BF00169313. [DOI] [PubMed] [Google Scholar]


