Abstract
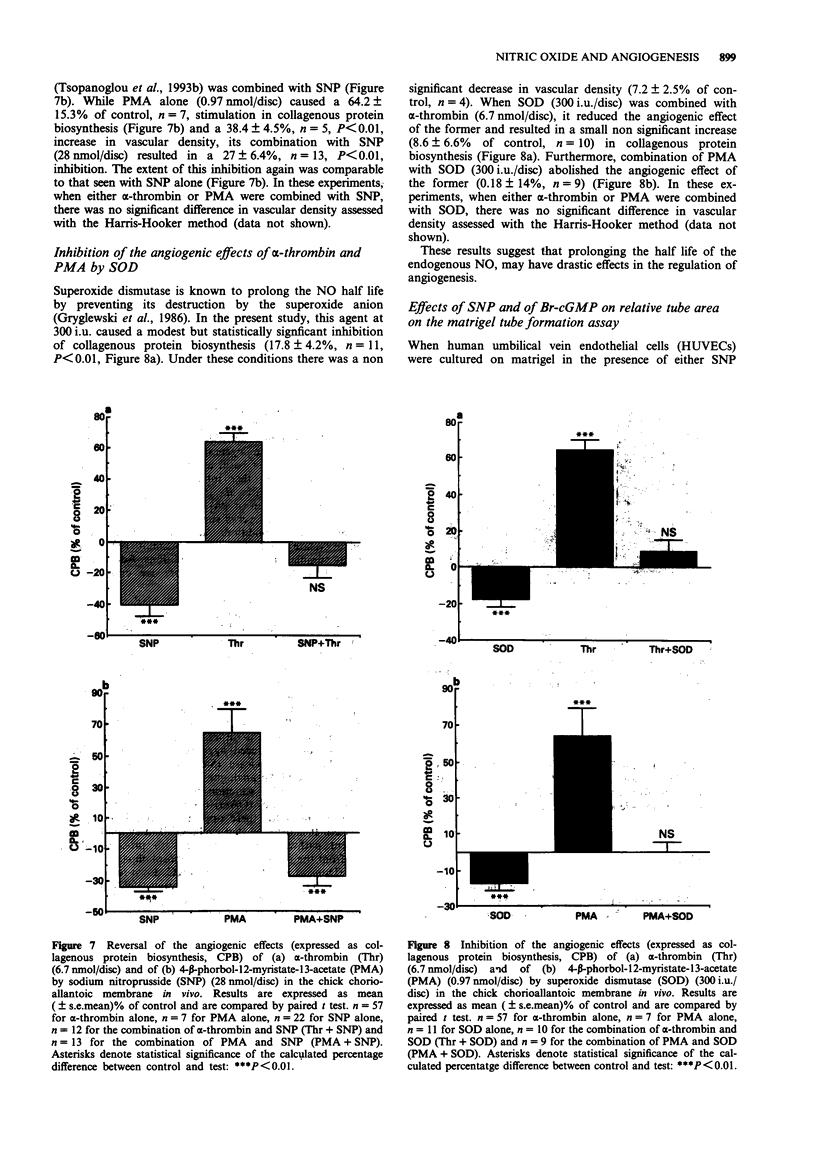
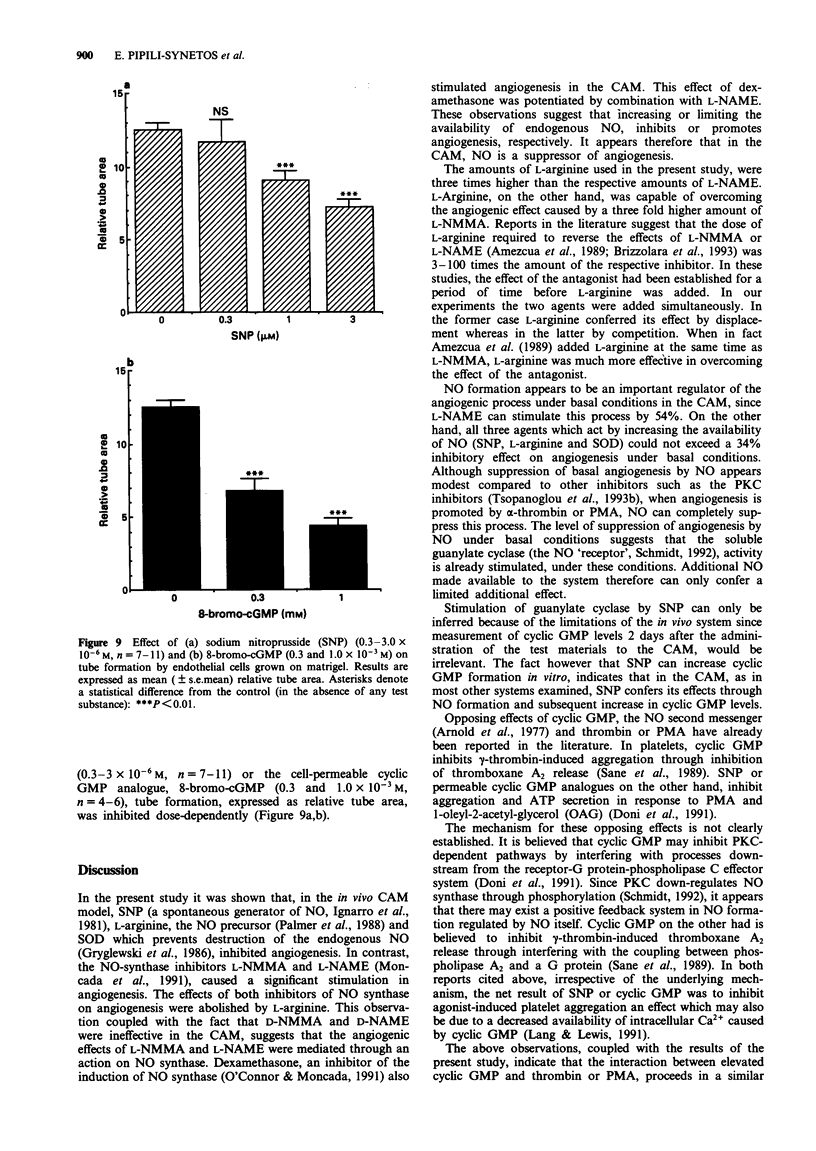
1. The involvement of nitric oxide (NO) in the regulation of angiogenesis was examined in the in vivo system of the chorioallantoic membrane (CAM) of the chick embryo and in the matrigel tube formation assay. 2. Sodium nitroprusside (SNP) (0.37-28 nmol/disc), which releases NO spontaneously, caused a dose-dependent inhibition of angiogenesis in the CAM in vivo and reversed completely the angiogenic effects of alpha-thrombin (6.7 nmol/disc) and the protein kinase C (PKC) activator 4-beta-phorbol-12-myristate-13-acetate (PMA) (0.97 nmol/disc). In addition, SNP (28 x 10(-6) M) stimulated the release of guanosine 3'-5'-cyclic monophosphate (cyclic GMP) from the CAM in vitro. 3. In the matrigel tube formation assay, an in vitro assay of angiogenesis, both SNP (1-3 x 10(-6) M) and the cell permeable cyclic GMP analogue, Br-cGMP (0.3-1.0 x 10(-3) M) reduced tube formation. 4. The inhibitors of NO synthase, NG-monomethyl-L-arginine (L-NMMA) (3.8-102 nmol/disc) and NG-nitro-L-arginine methylester (L-NAME) (1.3-34.2 nmol/disc) stimulated angiogenesis in the CAM in vivo, in a dose-dependent fashion. D-NMMA and D-NAME on the other hand had no effect on angiogenesis in this system. 5. L-Arginine (10.9 nmol/disc), although it had a modest antiangiogenic effect by itself, was capable of abolishing the angiogenic effects of L-NMMA (34.2 nmol/disc) and of L-NAME (3.8 nmol/disc). 6. Dexamethasone, an inhibitor of the induction of NO synthase, at 0.2-116.1 nmol/disc, stimulated angiogenesis in the CAM, whereas at 348.4-1161 nmol/disc it inhibited this process. Combination of 38.7 nmol/disc dexamethasone with L-NAME (9.3 nmol/disc) resulted in a potentiation of the angiogenic effect of the former. It appears therefore that both the constitutive and the inducible NO synthase may contribute to the NO-mediated inhibition of angiogenesis.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amezcua J. L., Palmer R. M., de Souza B. M., Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989 Aug;97(4):1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzolara A. L., Crowe R., Burnstock G. Evidence for the involvement of both ATP and nitric oxide in non-adrenergic, non-cholinergic inhibitory neurotransmission in the rabbit portal vein. Br J Pharmacol. 1993 Jul;109(3):606–608. doi: 10.1111/j.1476-5381.1993.tb13614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Doni M. G., Deana R., Padoin E., Ruzzene M., Alexandre A. Platelet activation by diacylglycerol or ionomycin is inhibited by nitroprusside. Biochim Biophys Acta. 1991 Sep 24;1094(3):323–329. doi: 10.1016/0167-4889(91)90093-d. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Ofosu F. A., Moon D. G., Maraganore J. M. Thrombin structure and function: why thrombin is the primary target for antithrombotics. Blood Coagul Fibrinolysis. 1991 Feb;2(1):69–75. [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Förstermann U., Schmidt H. H., Pollock J. S., Sheng H., Mitchell J. A., Warner T. D., Nakane M., Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991 Oct 24;42(10):1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Inhibition of rat mesangial cell mitogenesis by nitric oxide-generating vasodilators. Am J Physiol. 1989 Jul;257(1 Pt 2):F60–F66. doi: 10.1152/ajprenal.1989.257.1.F60. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
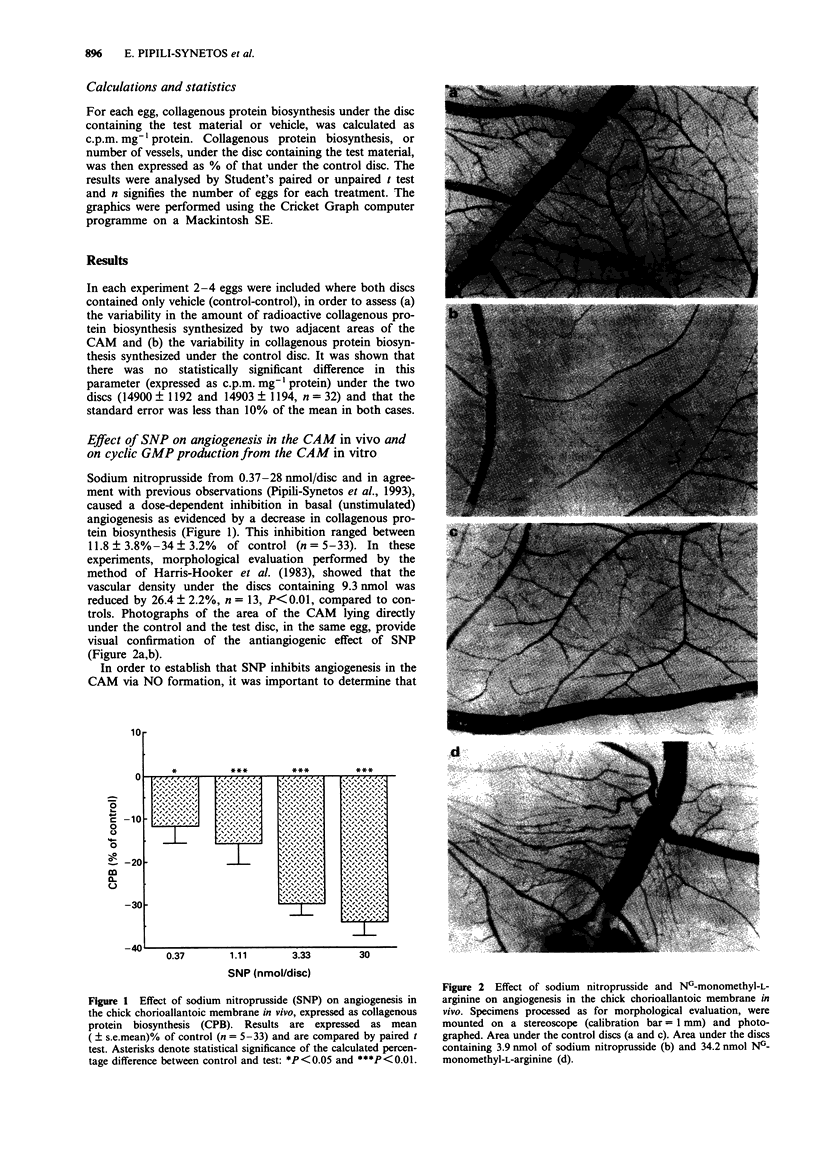
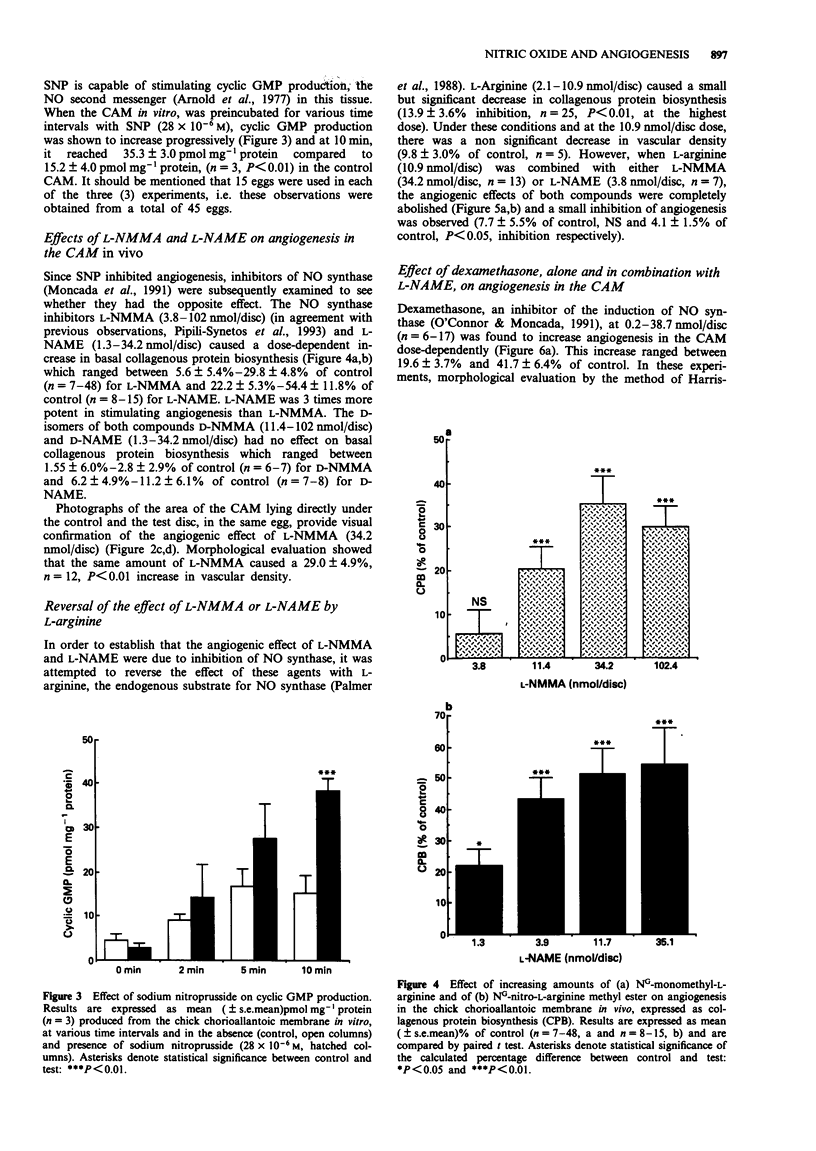
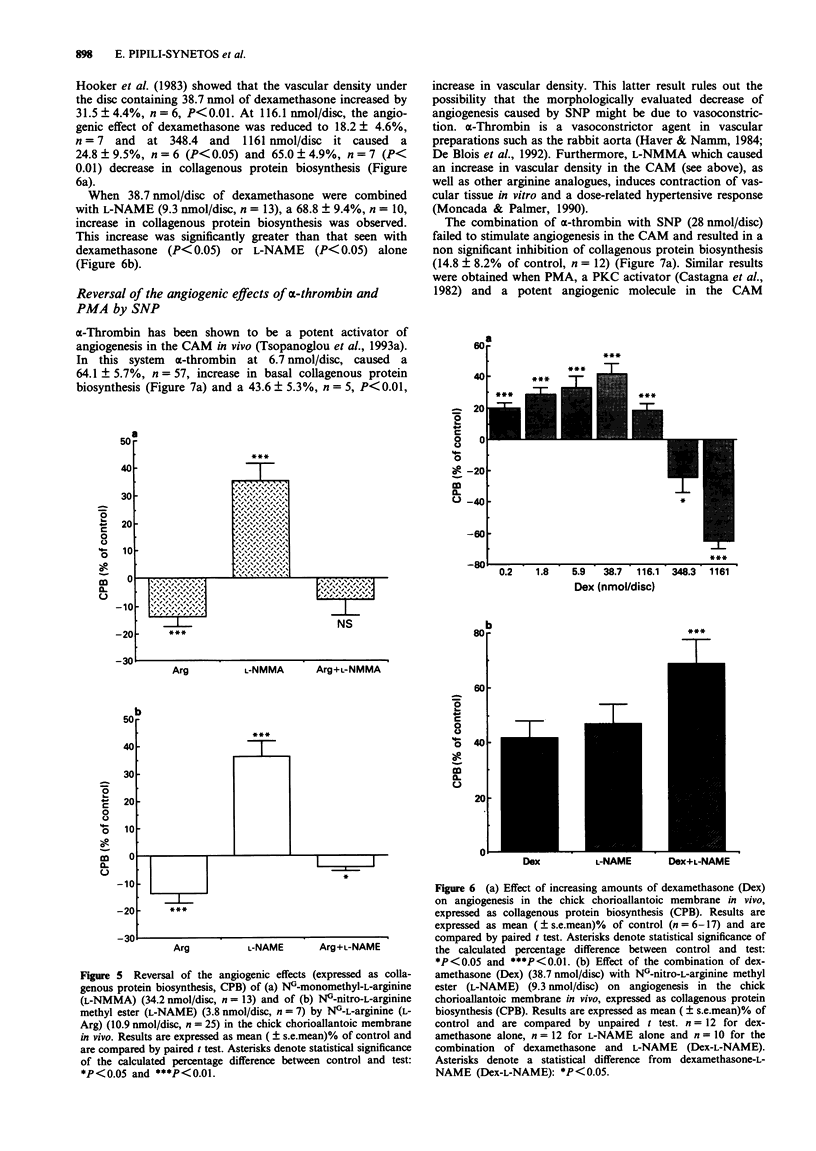
- Harris-Hooker S. A., Gajdusek C. M., Wight T. N., Schwartz S. M. Neovascular responses induced by cultured aortic endothelial cells. J Cell Physiol. 1983 Mar;114(3):302–310. doi: 10.1002/jcp.1041140308. [DOI] [PubMed] [Google Scholar]
- Haver V. M., Namm D. H. Characterization of the thrombin-induced contraction of vascular smooth muscle. Blood Vessels. 1984;21(2):53–63. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hu D. E., Hori Y., Fan T. P. Interleukin-8 stimulates angiogenesis in rats. Inflammation. 1993 Apr;17(2):135–143. doi: 10.1007/BF00916100. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Kubota Y., Kleinman H. K., Martin G. R., Lawley T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988 Oct;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D., Lewis M. J. Inhibition of inositol 1,4,5-trisphosphate formation by cyclic GMP in cultured aortic endothelial cells of the pig. Br J Pharmacol. 1991 Jan;102(1):277–281. doi: 10.1111/j.1476-5381.1991.tb12166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Mahadevan V., Hart I. R., Lewis G. P. Factors influencing blood supply in wound granuloma quantitated by a new in vivo technique. Cancer Res. 1989 Jan 15;49(2):415–419. [PubMed] [Google Scholar]
- Maragoudakis M. E., Panoutsacopoulou M., Sarmonika M. Rate of basement membrane biosynthesis as an index to angiogenesis. Tissue Cell. 1988;20(4):531–539. doi: 10.1016/0040-8166(88)90055-9. [DOI] [PubMed] [Google Scholar]
- Maragoudakis M. E., Sarmonika M., Panoutsacopoulou M. Antiangiogenic action of heparin plus cortisone is associated with decreased collagenous protein synthesis in the chick chorioallantoic membrane system. J Pharmacol Exp Ther. 1989 Nov;251(2):679–682. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nakaki T., Nakayama M., Kato R. Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. 1990 Dec 15;189(6):347–353. doi: 10.1016/0922-4106(90)90031-r. [DOI] [PubMed] [Google Scholar]
- Nakamura O., Ueki K., Hibi T., Shitara N., Matsutani M., Takakura K., Oikawa T. [Inhibition of neovascularization and tumor growth by dexamethasone]. No To Shinkei. 1992 Jan;44(1):37–41. [PubMed] [Google Scholar]
- O'Connor K. J., Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase and the related cell damage in adenocarcinoma cells. Biochim Biophys Acta. 1991 Oct 21;1097(3):227–231. doi: 10.1016/0925-4439(91)90040-g. [DOI] [PubMed] [Google Scholar]
- Page C. P. The involvement of platelets in non-thrombotic processes. Trends Pharmacol Sci. 1988 Feb;9(2):66–71. doi: 10.1016/0165-6147(88)90120-4. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Pipili-Synetos E., Sakkoula E., Maragoudakis M. E. Nitric oxide is involved in the regulation of angiogenesis. Br J Pharmacol. 1993 Apr;108(4):855–857. doi: 10.1111/j.1476-5381.1993.tb13476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles F. R., Edwards R. L. Activation of blood coagulation in cancer: Trousseau's syndrome revisited. Blood. 1983 Jul;62(1):14–31. [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Sane D. C., Bielawska A., Greenberg C. S., Hannun Y. A. Cyclic GMP analogs inhibit gamma thrombin-induced arachidonic acid release in human platelets. Biochem Biophys Res Commun. 1989 Dec 15;165(2):708–714. doi: 10.1016/s0006-291x(89)80024-5. [DOI] [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Schmidt H. H. NO., CO and .OH. Endogenous soluble guanylyl cyclase-activating factors. FEBS Lett. 1992 Jul 27;307(1):102–107. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- Schneemann M., Schoedon G., Frei K., Schaffner A. Immunovascular communication: activation and deactivation of murine endothelial cell nitric oxide synthase by cytokines. Immunol Lett. 1993 Feb;35(2):159–162. doi: 10.1016/0165-2478(93)90085-g. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Tsopanoglou N. E., Pipili-Synetos E., Maragoudakis M. E. Thrombin promotes angiogenesis by a mechanism independent of fibrin formation. Am J Physiol. 1993 May;264(5 Pt 1):C1302–C1307. doi: 10.1152/ajpcell.1993.264.5.C1302. [DOI] [PubMed] [Google Scholar]
- Wolff J. E., Guerin C., Laterra J., Bressler J., Indurti R. R., Brem H., Goldstein G. W. Dexamethasone reduces vascular density and plasminogen activator activity in 9L rat brain tumors. Brain Res. 1993 Feb 26;604(1-2):79–85. doi: 10.1016/0006-8993(93)90354-p. [DOI] [PubMed] [Google Scholar]
- Wolff J. E., Laterra J., Goldstein G. W. Steroid inhibition of neural microvessel morphogenesis in vitro: receptor mediation and astroglial dependence. J Neurochem. 1992 Mar;58(3):1023–1032. doi: 10.1111/j.1471-4159.1992.tb09357.x. [DOI] [PubMed] [Google Scholar]
- deBlois D., Drapeau G., Petitclerc E., Marceau F. Synergism between the contractile effect of epidermal growth factor and that of des-Arg9-bradykinin or of alpha-thrombin in rabbit aortic rings. Br J Pharmacol. 1992 Apr;105(4):959–967. doi: 10.1111/j.1476-5381.1992.tb09085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]