Abstract
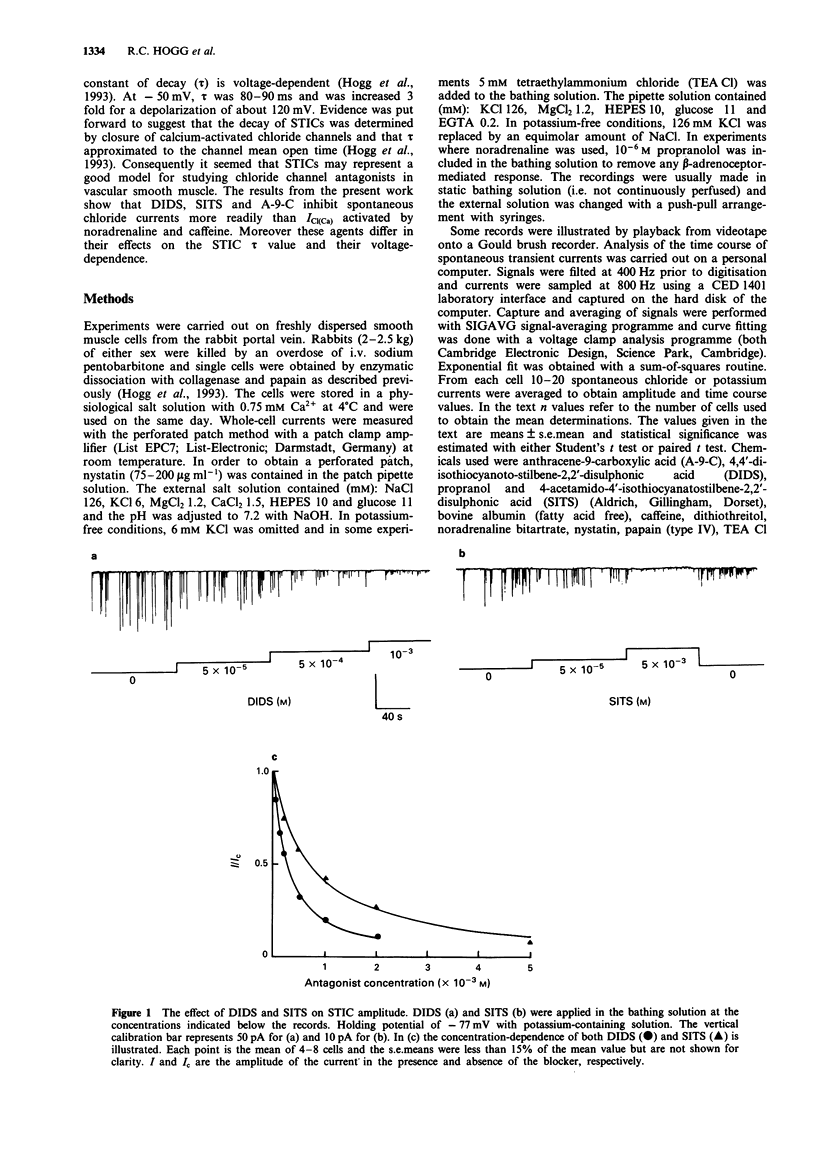
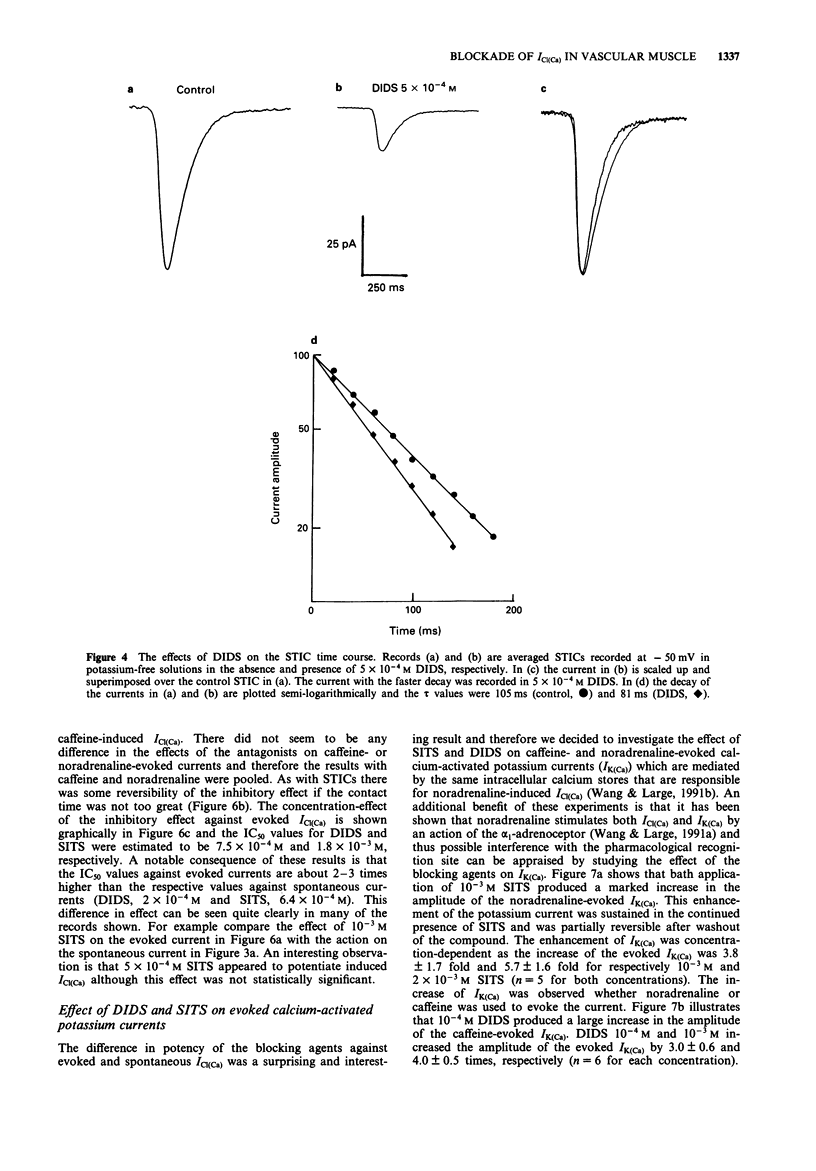
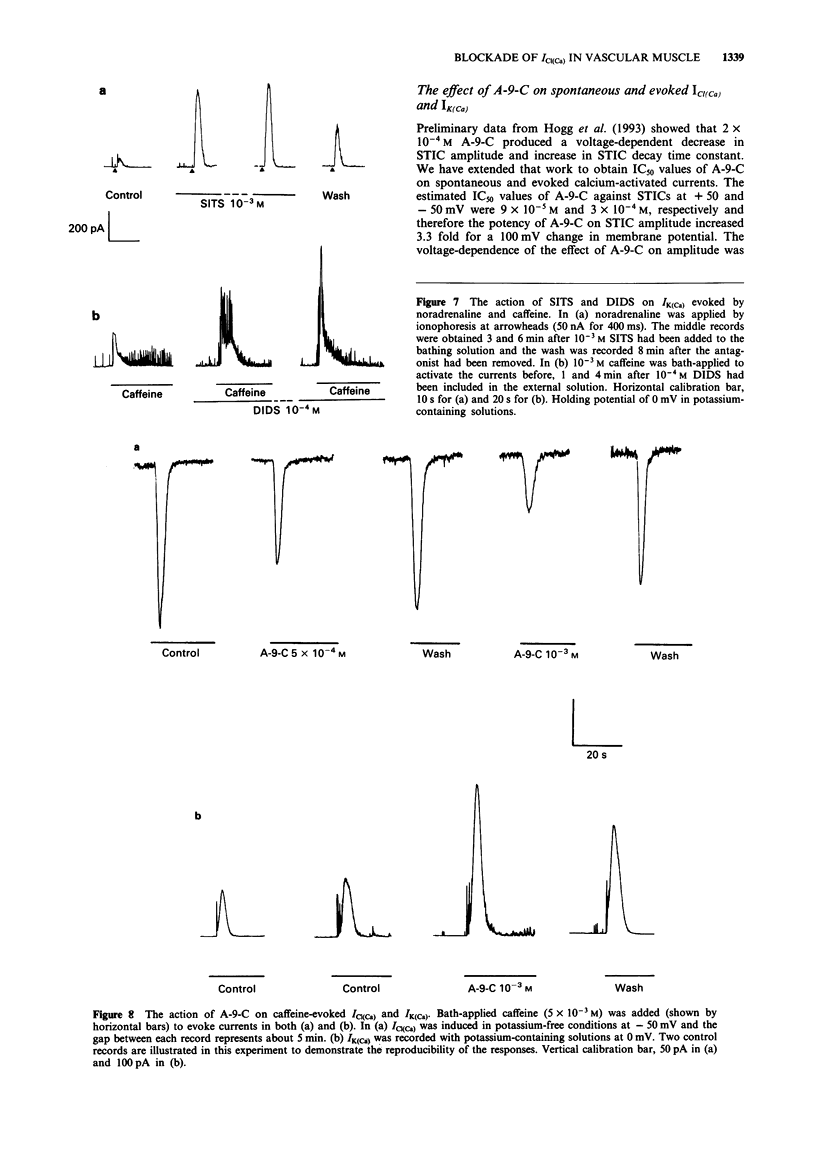
1. The effects of some chloride channel antagonists were studied on the calcium-activated chloride current (ICl(Ca)) in smooth muscle cells from the rabbit portal vein with the perforated patch technique. 2. 4-Acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid (SITS) and 4,4'-diisothiocyanato-stilbene-2,2'-disulphonic acid (DIDS) reduced the amplitude of spontaneous transient inward currents (STICs, calcium-activated chloride currents) in a concentration-dependent manner. The concentrations required to reduce the amplitude by 50% (IC50) of STICs were 2.1 x 10(-4) M and 6.4 x 10(-4) M for DIDS and SITS, respectively. This effect was not voltage-dependent. 3. The time constant of decay of STICs (tau), which is voltage-dependent, was increased by about 30% by SITS and decreased by about 20% by DIDS. The effect of DIDS and SITS on tau was similar at holding potentials of -50 and +50 mV. 4. These compounds did not modify the characteristics of spontaneous transient outward currents (STOCs, calcium-activated potassium currents). 5. DIDS and SITS decreased the amplitude of ICl(Ca) evoked by noradrenaline and caffeine less potently than STICs with IC50 values of 7.5 x 10(-4) M and 1.8 x 10(-3) M, respectively. 6. DIDS and SITS increased the calcium-activated potassium current (IK(Ca) evoked by noradrenaline and caffeine by 3-6 fold. 7. Anthracene-9-carboxylic acid (A-9-C) inhibited STICs in a voltage-dependent fashion and was about 3 fold more active at +50 mV than at -50 mV. A-9-C increased STIC tau and this effect was enhanced by depolarization.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbarali H. I., Giles W. R. Ca2+ and Ca(2+)-activated Cl- currents in rabbit oesophageal smooth muscle. J Physiol. 1993 Jan;460:117–133. doi: 10.1113/jphysiol.1993.sp019462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Potassium, chloride and non-selective cation conductances opened by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Apr;423:551–568. doi: 10.1113/jphysiol.1990.sp018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol. 1987 Dec;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A., Pacaud P., Loirand G., Mironneau C., Mironneau J. Pharmacological block of Ca(2+)-activated Cl- current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1991 Dec;419(6):553–558. doi: 10.1007/BF00370294. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden D. J., Krouse M. E., Law T., Wine J. J. Stilbenes stimulate T84 Cl- secretion by elevating Ca2+. Am J Physiol. 1993 Feb;264(2 Pt 1):G325–G333. doi: 10.1152/ajpgi.1993.264.2.G325. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Action of noradrenaline on single smooth muscle cells freshly dispersed from the rat anococcygeus muscle. J Physiol. 1987 Aug;389:513–525. doi: 10.1113/jphysiol.1987.sp016669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988 Oct;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane mechanism associated with muscarinic receptor activation in single cells freshly dispersed from the rat anococcygeus muscle. Br J Pharmacol. 1987 Oct;92(2):371–379. doi: 10.1111/j.1476-5381.1987.tb11333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T., Ritchie J. M. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- Harvey R. D. Effects of stilbenedisulfonic acid derivatives on the cAMP-regulated chloride current in cardiac myocytes. Pflugers Arch. 1993 Feb;422(5):436–442. doi: 10.1007/BF00375068. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Hogg R. C., Wang Q., Large W. A. Time course of spontaneous calcium-activated chloride currents in smooth muscle cells from the rabbit portal vein. J Physiol. 1993 May;464:15–31. doi: 10.1113/jphysiol.1993.sp019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Kasai M. Disulfonic stilbene derivatives open the Ca2+ release channel of sarcoplasmic reticulum. J Biochem. 1989 Sep;106(3):401–405. doi: 10.1093/oxfordjournals.jbchem.a122865. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Weight F. F. Patch-clamp study of the calcium-dependent chloride current in AtT-20 pituitary cells. J Neurophysiol. 1987 Dec;58(6):1431–1451. doi: 10.1152/jn.1987.58.6.1431. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Ohta T., Ito S., Nakazato Y. Chloride currents activated by caffeine in rat intestinal smooth muscle cells. J Physiol. 1993 Jun;465:149–162. doi: 10.1113/jphysiol.1993.sp019670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Hogg R. C., Large W. A. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. J Physiol. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Large W. A. Modulation of noradrenaline-induced membrane currents by papaverine in rabbit vascular smooth muscle cells. J Physiol. 1991 Aug;439:501–512. doi: 10.1113/jphysiol.1991.sp018678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Large W. A. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J Physiol. 1991 Apr;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]


