Abstract
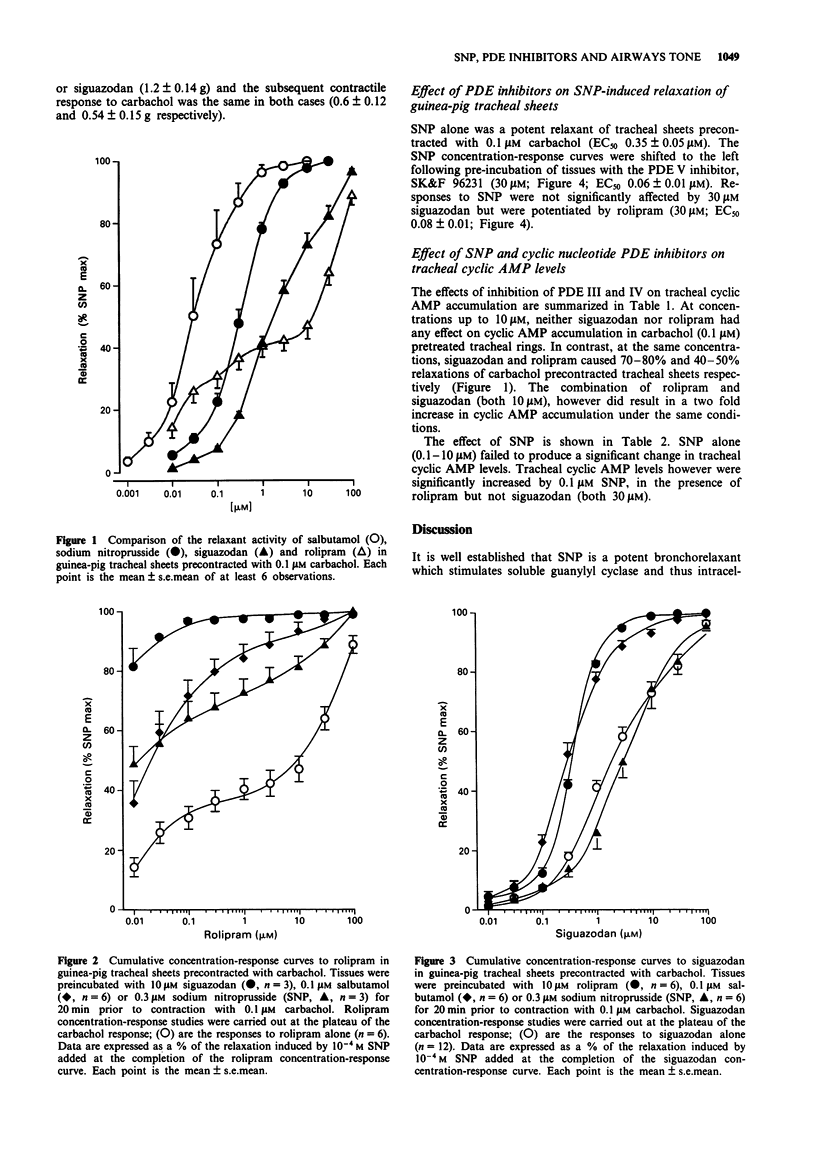
1. The effects of agents that elevate either cyclic AMP (the phosphodiesterase (PDE) III inhibitor siguazodan, salbutamol) or cyclic GMP (sodium nitroprusside (SNP)) on the relaxant activity of the PDE IV inhibitor, rolipram, were investigated in carbachol (0.1 microM) precontracted guinea-pig tracheal sheets. 2. Rolipram, siguazodan and SNP caused concentration-related reductions in tone of tissues precontracted with 0.1 microM carbachol (EC50 values 12.5; 2.73 and 0.35 microM respectively). Whilst the concentration-response relationship for the PDE III inhibitor, siguazodan, was monophasic that of the PDE IV inhibitor, rolipram, was biphasic. 3. The relaxant activity of rolipram was markedly enhanced in the presence of 10 microM siguazodan (EC50 < 0.01 microM), 0.1 microM salbutamol (EC50 0.03 microM) and 0.3 microM SNP (EC50 0.03 microM). In contrast, the relaxant activity of siguazodan was unaffected by SNP and only modestly enhanced by rolipram (10 microM) and salbutamol (0.1 microM). 4. The relaxant activity of SNP was enhanced by the PDE V inhibitor SK&F 96231 (30 microM: EC50 0.06 microM) and rolipram (30 microM, EC50 0.08 microM) but was unaffected by 30 microM siguazodan. 5. At concentrations up to 10 microM, neither siguazodan nor rolipram elevated tracheal cyclic AMP levels. However, the combination of 10 microM rolipram and siguazodan caused a two fold increase in the cyclic AMP content (from 2.19 to 4.36 pmol cyclic AMP mg-1 protein). SNP (0.1-10 microM) failed to produce a significant increase in tracheal cyclic AMP levels. At 0.1 microM the effect of SNP on tracheal cyclic AMP levels was significantly (P < 0.05) increased in the presence of rolipram but not siguadozan.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

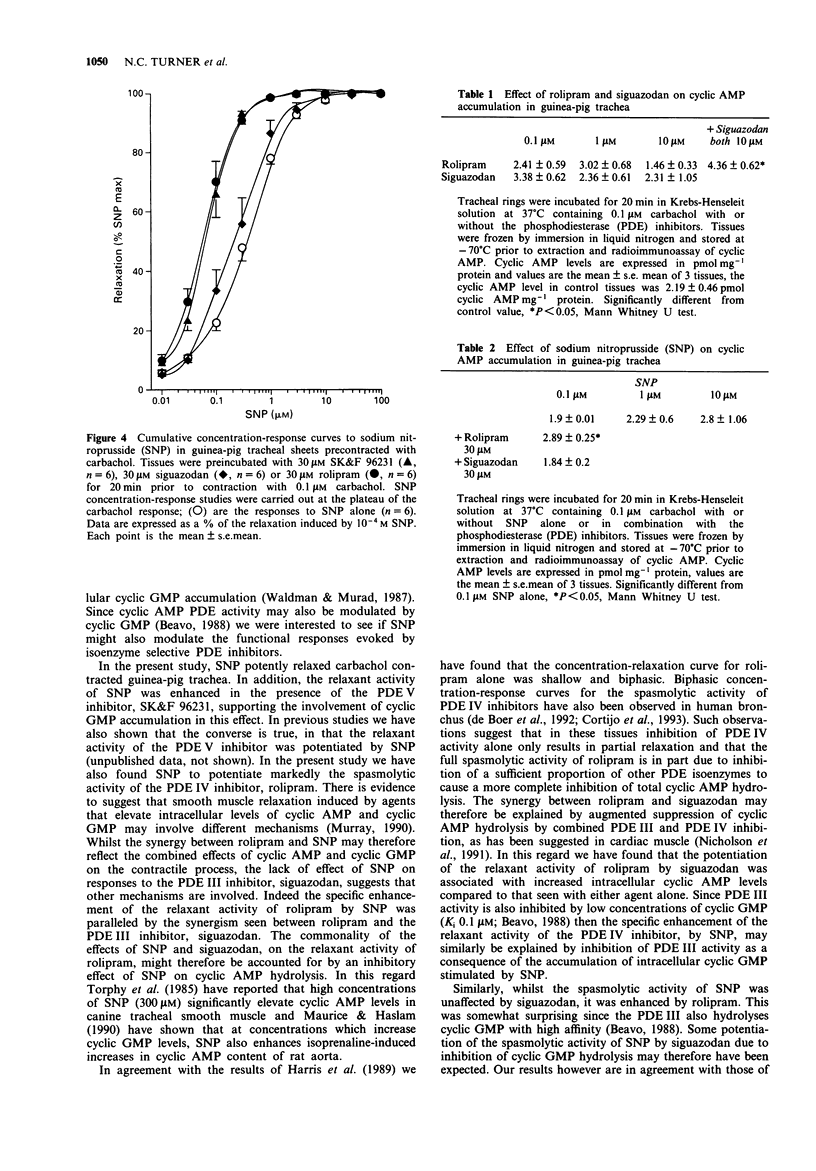


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cortijo J., Bou J., Beleta J., Cardelús I., Llenas J., Morcillo E., Gristwood R. W. Investigation into the role of phosphodiesterase IV in bronchorelaxation, including studies with human bronchus. Br J Pharmacol. 1993 Feb;108(2):562–568. doi: 10.1111/j.1476-5381.1993.tb12841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Inhibition of histamine-stimulated inositol phospholipid hydrolysis by agents which increase cyclic AMP levels in bovine tracheal smooth muscle. Br J Pharmacol. 1989 Jun;97(2):603–613. doi: 10.1111/j.1476-5381.1989.tb11992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Modulation of carbachol-induced inositol phosphate formation in bovine tracheal smooth muscle by cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol. 1990 Apr 15;39(8):1357–1363. doi: 10.1016/0006-2952(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Connell M. J., Ferguson E. W., Wallace A. M., Gordon R. J., Pagani E. D., Silver P. J. Role of low Km cyclic AMP phosphodiesterase inhibition in tracheal relaxation and bronchodilation in the guinea pig. J Pharmacol Exp Ther. 1989 Oct;251(1):199–206. [PubMed] [Google Scholar]
- Jiang H., Colbran J. L., Francis S. H., Corbin J. D. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem. 1992 Jan 15;267(2):1015–1019. [PubMed] [Google Scholar]
- Komas N., Lugnier C., Stoclet J. C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br J Pharmacol. 1991 Oct;104(2):495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. S., Lum B. K. Role of cyclic AMP in adrenergically-induced tracheal muscle relaxation. Arch Int Pharmacodyn Ther. 1983 Jan;261(1):36–50. [PubMed] [Google Scholar]
- Livi G. P., Kmetz P., McHale M. M., Cieslinski L. B., Sathe G. M., Taylor D. P., Davis R. L., Torphy T. J., Balcarek J. M. Cloning and expression of cDNA for a human low-Km, rolipram-sensitive cyclic AMP phosphodiesterase. Mol Cell Biol. 1990 Jun;10(6):2678–2686. doi: 10.1128/mcb.10.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann S. M., Miech R. P., Butcher F. R. Effects of isoproterenol, theophylline and carbachol on cyclic nucleotide levels and relaxation of bovine tracheal smooth muscle. Biochim Biophys Acta. 1977 Sep 29;499(2):238–250. doi: 10.1016/0304-4165(77)90006-x. [DOI] [PubMed] [Google Scholar]
- Maurice D. H., Haslam R. J. Nitroprusside enhances isoprenaline-induced increases in cAMP in rat aortic smooth muscle. Eur J Pharmacol. 1990 Dec 4;191(3):471–475. doi: 10.1016/0014-2999(90)94182-w. [DOI] [PubMed] [Google Scholar]
- Murray K. J. Cyclic AMP and mechanisms of vasodilation. Pharmacol Ther. 1990;47(3):329–345. doi: 10.1016/0163-7258(90)90060-f. [DOI] [PubMed] [Google Scholar]
- Murray K. J., Eden R. J., England P. J., Dolan J., Grimsditch D. C., Stutchbury C. A., Patel B., Reeves M. L., Worby A., Torphy T. J. Potential use of selective phosphodiesterase inhibitors in the treatment of asthma. Agents Actions Suppl. 1991;34:27–46. [PubMed] [Google Scholar]
- Nicholson C. D., Challiss R. A., Shahid M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol Sci. 1991 Jan;12(1):19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- Shahid M., van Amsterdam R. G., de Boer J., ten Berge R. E., Nicholson C. D., Zaagsma J. The presence of five cyclic nucleotide phosphodiesterase isoenzyme activities in bovine tracheal smooth muscle and the functional effects of selective inhibitors. Br J Pharmacol. 1991 Oct;104(2):471–477. doi: 10.1111/j.1476-5381.1991.tb12453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souness J. E., Carter C. M., Diocee B. K., Hassall G. A., Wood L. J., Turner N. C. Characterization of guinea-pig eosinophil phosphodiesterase activity. Assessment of its involvement in regulating superoxide generation. Biochem Pharmacol. 1991 Jul 25;42(4):937–945. doi: 10.1016/0006-2952(91)90056-b. [DOI] [PubMed] [Google Scholar]
- Torphy T. J., Burman M., Huang L. B., Tucker S. S. Inhibition of the low km cyclic AMP phosphodiesterase in intact canine trachealis by SK&F 94836: mechanical and biochemical responses. J Pharmacol Exp Ther. 1988 Sep;246(3):843–850. [PubMed] [Google Scholar]
- Torphy T. J., Cieslinski L. B. Characterization and selective inhibition of cyclic nucleotide phosphodiesterase isozymes in canine tracheal smooth muscle. Mol Pharmacol. 1990 Feb;37(2):206–214. [PubMed] [Google Scholar]
- Torphy T. J., Undem B. J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991 Jul;46(7):512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy T. J., Zheng C., Peterson S. M., Fiscus R. R., Rinard G. A., Mayer S. E. Inhibitory effect of methacholine on drug-induced relaxation, cyclic AMP accumulation, and cyclic AMP-dependent protein kinase activation in canine tracheal smooth muscle. J Pharmacol Exp Ther. 1985 May;233(2):409–417. [PubMed] [Google Scholar]
- Torphy T. J., Zhou H. L., Burman M., Huang L. B. Role of cyclic nucleotide phosphodiesterase isozymes in intact canine trachealis. Mol Pharmacol. 1991 Mar;39(3):376–384. [PubMed] [Google Scholar]
- Turner N. C., Wood L. J., Burns F. M., Gueremy T., Souness J. E. The effect of cyclic AMP and cyclic GMP phosphodiesterase inhibitors on the superoxide burst of guinea-pig peritoneal macrophages. Br J Pharmacol. 1993 Apr;108(4):876–883. doi: 10.1111/j.1476-5381.1993.tb13481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Wright C. D., Kuipers P. J., Kobylarz-Singer D., Devall L. J., Klinkefus B. A., Weishaar R. E. Differential inhibition of human neutrophil functions. Role of cyclic AMP-specific, cyclic GMP-insensitive phosphodiesterase. Biochem Pharmacol. 1990 Aug 15;40(4):699–707. doi: 10.1016/0006-2952(90)90304-4. [DOI] [PubMed] [Google Scholar]
- de Boer J., Philpott A. J., van Amsterdam R. G., Shahid M., Zaagsma J., Nicholson C. D. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br J Pharmacol. 1992 Aug;106(4):1028–1034. doi: 10.1111/j.1476-5381.1992.tb14451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


