Abstract
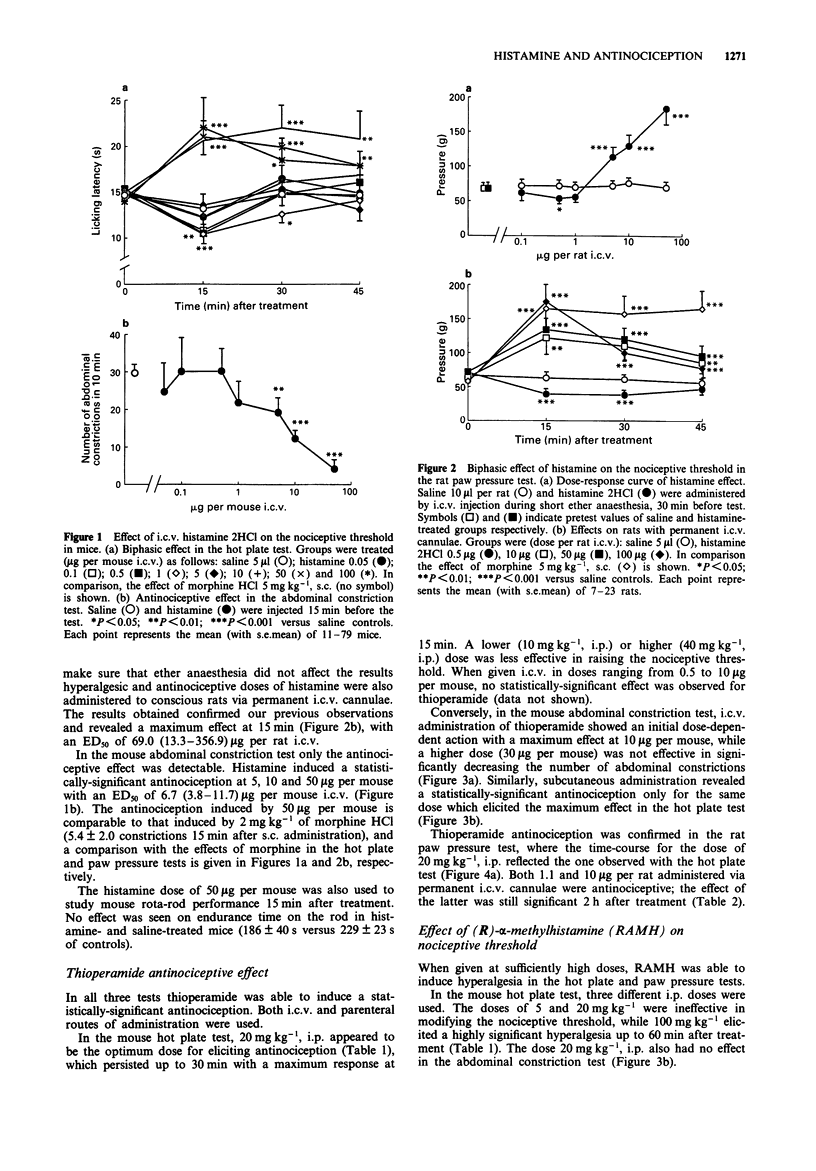
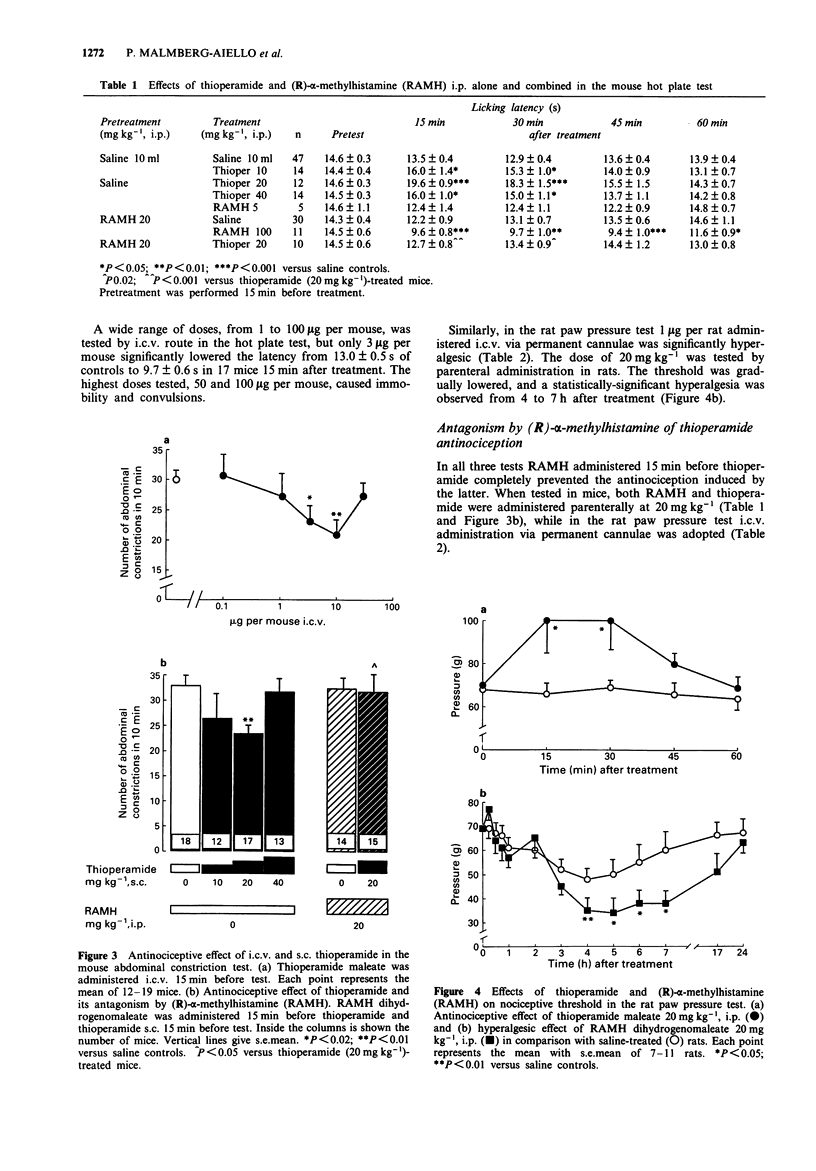
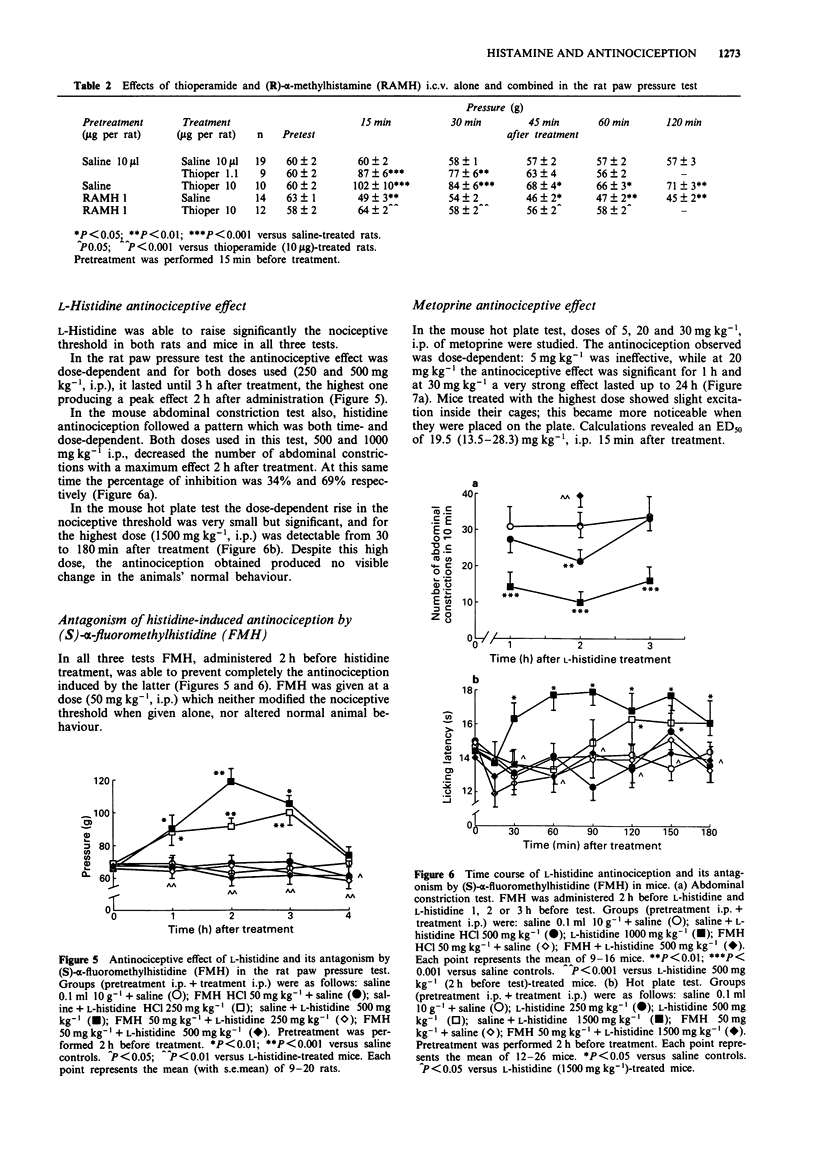
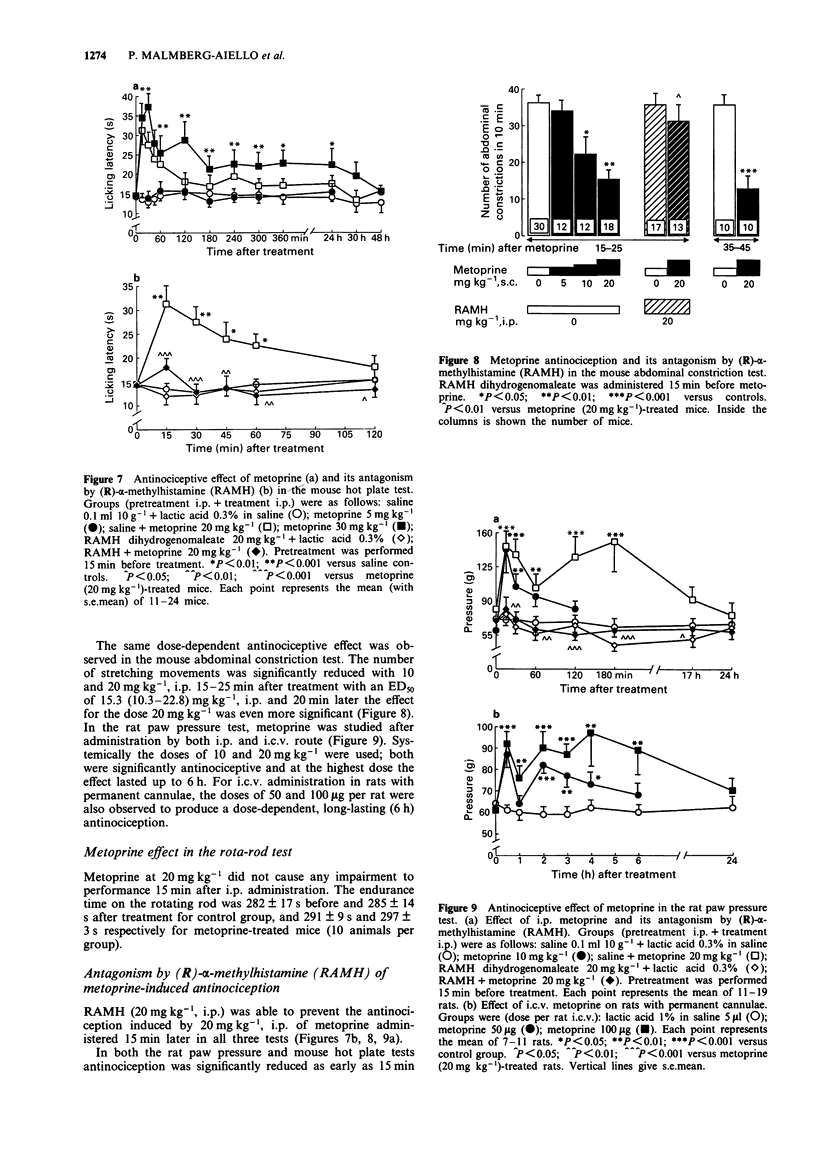
1. Effects of substances which are able to alter brain histamine levels on the nociceptive threshold were investigated in mice and rats by means of tests inducing three different kinds of noxious stimuli: mechanical (paw pressure), chemical (abdominal constriction) and thermal (hot plate). 2. A wide range of i.c.v. doses of histamine 2HCl was studied. Relatively high dose were dose-dependently antinociceptive in all three tests: 5-100 micrograms per rat in the paw pressure test, 5-50 micrograms per mouse in the abdominal constriction test and 50-100 micrograms per mouse in the hot plate test. Conversely, very low doses were hyperalgesic: 0.5 microgram per rat in the paw pressure test and 0.1-1 microgram per mouse in the hot plate test. In the abdominal constriction test no hyperalgesic effect was observed. 3. The histamine H3 antagonist, thioperamide maleate, elicited a weak but statistically significant dose-dependent antinociceptive effect by both parenteral (10-40 mg kg-1) and i.c.v. (1.1-10 micrograms per rat and 3.4-10 micrograms per mouse) routes. 4. The histamine H3 agonist, (R)-alpha-methylhistamine dihydrogenomaleate was hyperalgesic, with a rapid effect (15 min after treatment) following i.c.v. administration of 1 microgram per rat and 3 microgram per mouse, or i.p. administration of 100 mg kg-1 in mice. In rats 20 mg kg-1, i.p. elicited hyperalgesia only 4 h after treatment. 5. Thioperamide-induced antinociception was completely prevented by pretreatment with a non-hyperalgesic i.p. dose of (R)-alpha-methylhistamine in the mouse hot plate and abdominal constriction tests. Antagonism was also observed when both substances were administered i.c.v. in rats. 6. L-Histidine HCl dose-dependently induced a slowly occurring antinociception in all three tests. The doses of 250 and 500 mg kg-1, i.p. were effective in the rat paw pressure test, and those of 500 and 1500 mg kg-1, i.p. in the mouse hot plate test. In the mouse abdominal constriction test 500 and 1000 mg kg-1, i.p. showed their maximum effect 2 h after treatment. 7. The histamine N-methyltransferase inhibitor, metoprine, elicited a long-lasting, dose-dependent antinociception in all three tests by both i.p. (10-30 mg kg-1) and i.c.v. (50-100 micrograms per rat) routes. 8. To ascertain the mechanism of action of the antinociceptive effect of L-histidine and metoprine, the two substances were also studied in combination with the histamine synthesis inhibitor (S)-alpha-fluoromethylhistidine and with (R)-alpha-methylhistamine, respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou Y. Z., Adam H. M., Stephen W. R. Concentration of histamine in different parts of the brain and hypophysis of rabbit: effect of treatment with histidine, certain other amino acids and histamine. Br J Pharmacol. 1973 Aug;48(4):577–589. doi: 10.1111/j.1476-5381.1973.tb08244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaffer F. B., Verster F. S., Hall S., Long C. J., D'encarnacao P. A simple and inexpensive cannula technique for chemical stimulation of the brain. Physiol Behav. 1970 Jan;5(1):119–121. doi: 10.1016/0031-9384(70)90022-3. [DOI] [PubMed] [Google Scholar]
- Arrang J. M., Garbarg M., Lancelot J. C., Lecomte J. M., Pollard H., Robba M., Schunack W., Schwartz J. C. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987 May 14;327(6118):117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- Arrang J. M., Garbarg M., Schwartz J. C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983 Apr 28;302(5911):832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Arrang J. M., Garbarg M., Schwartz J. C. Autoinhibition of histamine synthesis mediated by presynaptic H3-receptors. Neuroscience. 1987 Oct;23(1):149–157. doi: 10.1016/0306-4522(87)90279-x. [DOI] [PubMed] [Google Scholar]
- BLASCHKO H., CHRUSCIEL T. L. The decarboxylation of amino acids related to tyrosine and their awakening action in reserpine-treated mice. J Physiol. 1960 May;151:272–284. doi: 10.1113/jphysiol.1960.sp006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini A., Ghelardini C., Fantetti L., Malcangio M., Malmberg-Aiello P., Giotti A. Role of muscarinic receptor subtypes in central antinociception. Br J Pharmacol. 1992 Jan;105(1):77–82. doi: 10.1111/j.1476-5381.1992.tb14213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini A., Renzi G., Galli A., Aiello P. M., Bartolini R. Eseroline: a new antinociceptive agent derived from physostigmine with opiate receptor agonist properties. Experimental in vivo and in vitro studies on cats and rodents. Neurosci Lett. 1981 Sep 1;25(2):179–183. doi: 10.1016/0304-3940(81)90328-1. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Beaumont A., Hernandez J. F., Chaillet P., Crine P., Roques B. P. Irreversible photolabeling of active site of neutral endopeptidase-24.11 "enkephalinase" by azidothiorphan and [14C]-azidothiorphan. Mol Pharmacol. 1987 Nov;32(5):594–599. [PubMed] [Google Scholar]
- Bhattacharya S. K., Parmar S. S. Antinociceptive effect of intracerebroventricularly administered histamine in rats. Res Commun Chem Pathol Pharmacol. 1985 Jul;49(1):125–136. [PubMed] [Google Scholar]
- Braga P. C., Sibilia V., Guidobono E., Pecile A., Netti C. Electrophysiological correlates for antinociceptive effects of histamine after intracerebral administration to the rat. Neuropharmacology. 1992 Sep;31(9):937–941. doi: 10.1016/0028-3908(92)90133-a. [DOI] [PubMed] [Google Scholar]
- Buckett W. R. Irreversible inhibitors of GABA transaminase induce antinociceptive effects and potentiate morphine. Neuropharmacology. 1980 Aug;19(8):715–722. doi: 10.1016/0028-3908(80)90062-3. [DOI] [PubMed] [Google Scholar]
- Chipkin R. E., Berger J. G., Billard W., Iorio L. C., Chapman R., Barnett A. Pharmacology of SCH 34826, an orally active enkephalinase inhibitor analgesic. J Pharmacol Exp Ther. 1988 Jun;245(3):829–838. [PubMed] [Google Scholar]
- Chung Y. H., Miyake H., Kamei C., Tasaka K. Analgesic effect of histamine induced by intracerebral injection into mice. Agents Actions. 1984 Oct;15(3-4):137–142. doi: 10.1007/BF01972339. [DOI] [PubMed] [Google Scholar]
- Costa L. G., Murphy S. D. Cholinergic and opiate involvement in the antinociceptive effect of diisopropylfluorophosphate. Pharmacol Biochem Behav. 1986 Mar;24(3):733–736. doi: 10.1016/0091-3057(86)90582-4. [DOI] [PubMed] [Google Scholar]
- Duch D. S., Bowers S. W., Nichol C. A. Elevation of brain histamine levels by diaminopyrimidine inhibitors of histamine N-methyl transferase. Biochem Pharmacol. 1978 May 15;27(10):1507–1509. doi: 10.1016/0006-2952(78)90109-0. [DOI] [PubMed] [Google Scholar]
- Garbarg M., Barbin G., Rodergas E., Schwartz J. C. Inhibition of histamine synthesis in brain by alpha-fluoromethylhistidine, a new irreversible inhibitor: in vitro and in vivo studies. J Neurochem. 1980 Nov;35(5):1045–1052. doi: 10.1111/j.1471-4159.1980.tb07858.x. [DOI] [PubMed] [Google Scholar]
- Garbarg M., Tuong M. D., Gros C., Schwartz J. C. Effects of histamine H3-receptor ligands on various biochemical indices of histaminergic neuron activity in rat brain. Eur J Pharmacol. 1989 May 2;164(1):1–11. doi: 10.1016/0014-2999(89)90225-2. [DOI] [PubMed] [Google Scholar]
- Ghelardini C., Malmberg-Aiello P., Giotti A., Malcangio M., Bartolini A. Investigation into atropine-induced antinociception. Br J Pharmacol. 1990 Sep;101(1):49–54. doi: 10.1111/j.1476-5381.1990.tb12087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick S. D., Crane L. A. Opiate-like and abstinence-like effects of intracerebral histamine administration in rats. Nature. 1978 Jun 15;273(5663):547–549. doi: 10.1038/273547a0. [DOI] [PubMed] [Google Scholar]
- Green P. G., Kitchen I. Di-isopropylfluorophosphate induced antinociception and its interactions with opioid drugs in the rat. Toxicology. 1986 Dec 15;42(2-3):275–280. doi: 10.1016/0300-483x(86)90015-6. [DOI] [PubMed] [Google Scholar]
- Greenberg R., O'Keefe E. H. Thiorphan potentiation of stress-induced analgesia in the mouse. Life Sci. 1982 Sep 20;31(12-13):1185–1188. doi: 10.1016/0024-3205(82)90338-1. [DOI] [PubMed] [Google Scholar]
- HALEY T. J., MCCORMICK W. G. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957 Mar;12(1):12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough L. B., Khandelwal J. K., Green J. P. Inhibition of brain histamine metabolism by metoprine. Biochem Pharmacol. 1986 Jan 15;35(2):307–310. doi: 10.1016/0006-2952(86)90530-7. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Oishi R., Nishibori M., Saeki K. Characterization of histamine release from the rat hypothalamus as measured by in vivo microdialysis. J Neurochem. 1991 Mar;56(3):769–774. doi: 10.1111/j.1471-4159.1991.tb01990.x. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Oishi R., Nishibori M., Saeki K. Effects of nociceptive stimuli on brain histamine dynamics. Jpn J Pharmacol. 1989 Apr;49(4):449–454. doi: 10.1254/jjp.49.449. [DOI] [PubMed] [Google Scholar]
- Jones S. L., Gebhart G. F. Characterization of coeruleospinal inhibition of the nociceptive tail-flick reflex in the rat: mediation by spinal alpha 2-adrenoceptors. Brain Res. 1986 Feb 5;364(2):315–330. doi: 10.1016/0006-8993(86)90844-9. [DOI] [PubMed] [Google Scholar]
- Kollonitsch J., Perkins L. M., Patchett A. A., Doldouras G. A., Marburg S., Duggan D. E., Maycock A. L., Aster S. D. Selective inhibitors of biosynthesis of aminergic neurotransmitters. Nature. 1978 Aug 31;274(5674):906–908. doi: 10.1038/274906a0. [DOI] [PubMed] [Google Scholar]
- Kuribara H., Higuchi Y., Tadokoro S. Effects of central depressants on rota-rod and traction performances in mice. Jpn J Pharmacol. 1977 Feb;27(1):117–126. doi: 10.1254/jjp.27.117. [DOI] [PubMed] [Google Scholar]
- Leighton G. E., Rodriguez R. E., Hill R. G., Hughes J. kappa-Opioid agonists produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br J Pharmacol. 1988 Mar;93(3):553–560. doi: 10.1111/j.1476-5381.1988.tb10310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Gordon N. C., Fields H. L. Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature. 1979 Apr 19;278(5706):740–741. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- Liebman J. M., Pastor G. Antinociceptive effects of baclofen and muscimol upon intraventricular administration. Eur J Pharmacol. 1980 Feb 8;61(3):225–230. doi: 10.1016/0014-2999(80)90124-7. [DOI] [PubMed] [Google Scholar]
- Maeyama K., Watanabe T., Taguchi Y., Yamatodani A., Wada H. Effect of alpha-fluoromethylhistidine, a suicide inhibitor of histidine decarboxylase, on histamine levels in mouse tissues. Biochem Pharmacol. 1982 Jul 15;31(14):2367–2370. doi: 10.1016/0006-2952(82)90531-7. [DOI] [PubMed] [Google Scholar]
- Maeyama K., Watanabe T., Yamatodani A., Taguchi Y., Kambe H., Wada H. Effect of alpha-fluoromethylhistidine on the histamine content of the brain of W/Wv mice devoid of mast cells: turnover of brain histamine. J Neurochem. 1983 Jul;41(1):128–134. doi: 10.1111/j.1471-4159.1983.tb11823.x. [DOI] [PubMed] [Google Scholar]
- Malcangio M., Ghelardini C., Giotti A., Malmberg-Aiello P., Bartolini A. CGP 35348, a new GABAB antagonist, prevents antinociception and muscle-relaxant effect induced by baclofen. Br J Pharmacol. 1991 Jun;103(2):1303–1308. doi: 10.1111/j.1476-5381.1991.tb09784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M., Malmberg-Aiello P., Giotti A., Ghelardini C., Bartolini A. Desensitization of GABAB receptors and antagonism by CGP 35348, prevent bicuculline- and picrotoxin-induced antinociception. Neuropharmacology. 1992 Aug;31(8):783–791. doi: 10.1016/0028-3908(92)90042-n. [DOI] [PubMed] [Google Scholar]
- Metys J., Wagner N., Metysová J., Herz A. Studies on the central antinociceptive action of cholinomimetic agents. Int J Neuropharmacol. 1969 Sep;8(5):413–425. doi: 10.1016/0028-3908(69)90058-6. [DOI] [PubMed] [Google Scholar]
- O'Callaghan J. P., Holtzman S. G. Prenatal administration of morphine to the rat: tolerance to the analgesic effect of morphine in the offspring. J Pharmacol Exp Ther. 1976 Jun;197(3):533–544. [PubMed] [Google Scholar]
- Oishi R., Itoh Y., Nishibori M., Saeki K. Effects of the histamine H3-agonist (R)-alpha-methylhistamine and the antagonist thioperamide on histamine metabolism in the mouse and rat brain. J Neurochem. 1989 May;52(5):1388–1392. doi: 10.1111/j.1471-4159.1989.tb09184.x. [DOI] [PubMed] [Google Scholar]
- Oishi R., Nishibori M., Saeki K. Regional differences in the turnover of neuronal histamine in the rat brain. Life Sci. 1984 Feb 13;34(7):691–699. doi: 10.1016/0024-3205(84)90234-0. [DOI] [PubMed] [Google Scholar]
- Oluyomi A. O., Hart S. L. Involvement of histamine in naloxone-resistant and naloxone-sensitive models of swim stress-induced antinociception in the mouse. Neuropharmacology. 1991 Sep;30(9):1021–1027. doi: 10.1016/0028-3908(91)90115-r. [DOI] [PubMed] [Google Scholar]
- Panula P., Pirvola U., Auvinen S., Airaksinen M. S. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28(3):585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Parolaro D., Patrini G., Massi P., Mainardi P., Giagnoni G., Sala M., Gori E. Histamine as a central modulator of rat intestinal transit. J Pharmacol Exp Ther. 1989 Apr;249(1):324–328. [PubMed] [Google Scholar]
- Samanin R., Valzelli L. Increase of morphine-induced analgesia by stimulation of the nucleus raphe dorsalis. Eur J Pharmacol. 1971 Nov-Dec;16(3):298–302. doi: 10.1016/0014-2999(71)90030-6. [DOI] [PubMed] [Google Scholar]
- Sawynok J., LaBella F. S. On the involvement of GABA in the analgesia produced by baclofen, muscimol and morphine. Neuropharmacology. 1982 May;21(5):397–403. doi: 10.1016/0028-3908(82)90022-3. [DOI] [PubMed] [Google Scholar]
- Schayer R. W., Reilly M. A. Formation and fate of histamine in rat and mouse brain. J Pharmacol Exp Ther. 1973 Jan;184(1):33–40. [PubMed] [Google Scholar]
- Schwartz J. C., Arrang J. M., Garbarg M., Pollard H. Plenary lecture. A third histamine receptor subtype: characterisation, localisation and functions of the H3-receptor. Agents Actions. 1990 Apr;30(1-2):13–23. doi: 10.1007/BF01968988. [DOI] [PubMed] [Google Scholar]
- Schwartz J. C., Lampart C., Rose C. Histamine formation in rat brain in vivo: effects of histidine loads. J Neurochem. 1972 Mar;19(3):801–810. doi: 10.1111/j.1471-4159.1972.tb01394.x. [DOI] [PubMed] [Google Scholar]
- Schwartz J. C., Lampart C., Rose C., Rehault M. C., Bischoff S., Pollard H. Histamine formation in rat brain during development. J Neurochem. 1971 Sep;18(9):1787–1789. doi: 10.1111/j.1471-4159.1971.tb03757.x. [DOI] [PubMed] [Google Scholar]
- Schwartz J. C., Pollard H., Bischoff S., Rehault M. C., Verdiere-Sahuque M. Catabolism of 3 H-histamine in the rat brain after intracisternal administration. Eur J Pharmacol. 1971 Nov-Dec;16(3):326–335. doi: 10.1016/0014-2999(71)90035-5. [DOI] [PubMed] [Google Scholar]
- Sheiner J. B., Morris P., Anderson G. H. Food intake suppression by histidine. Pharmacol Biochem Behav. 1985 Nov;23(5):721–726. doi: 10.1016/0091-3057(85)90061-9. [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Snyder S. H. Dynamics of the regulation of histamine levels in mouse brain. J Neurochem. 1972 Feb;19(2):341–354. doi: 10.1111/j.1471-4159.1972.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Michel A. D., Kilpatrick G. J. In vivo occupancy of histamine H3 receptors by thioperamide and (R)-alpha-methylhistamine measured using histamine turnover and an ex vivo labeling technique. Biochem Pharmacol. 1992 Oct 6;44(7):1261–1267. doi: 10.1016/0006-2952(92)90524-m. [DOI] [PubMed] [Google Scholar]
- Tyers M. B. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol. 1980 Jul;69(3):503–512. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf J. F., Bast A., Bijloo G. J., Van der Vliet A., Timmerman H. HA autoreceptor assay with superfused slices of rat brain cortex and electrical stimulation. Eur J Pharmacol. 1987 Jun 19;138(2):199–206. doi: 10.1016/0014-2999(87)90433-x. [DOI] [PubMed] [Google Scholar]
- Wada H., Inagaki N., Itowi N., Yamatodani A. Histaminergic neuron system in the brain: distribution and possible functions. Brain Res Bull. 1991 Sep-Oct;27(3-4):367–370. doi: 10.1016/0361-9230(91)90126-5. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Noueihed R. The physiology and pharmacology of spinal opiates. Annu Rev Pharmacol Toxicol. 1985;25:433–462. doi: 10.1146/annurev.pa.25.040185.002245. [DOI] [PubMed] [Google Scholar]


