Abstract
1. The presence of A1 adenosine receptors in CHO-K1 cells transfected with the human brain A1 sequence was confirmed by ligand binding studies using 8-cyclopentyl-[3H] 1,3-dipropylxanthine ([3H]-DPCPX). 2. Alterations in intracellular calcium ([Ca2+]i) were measured with the calcium-sensitive dye, fura-2. 3. N6-cyclopentyladenosine (CPA), the selective A1 agonist, and 5'-N-ethylcarboxaminoadenosine (NECA), a relatively non-selective adenosine receptor agonist, elicited rapid, biphasic increases in [Ca2+]i which involved both mobilisation from intracellular stores and calcium entry. 4. The calcium response to CPA was significantly inhibited by the selective A1 antagonist DPCPX. The non-selective adenosine receptor, xanthine amino congener (XAC), was less potent. 5. The calcium response to CPA was completely prevented by pretreatment of the cells with pertussis toxin implicating the involvement of Gi in the receptor-mediated response. 6. In summary, we present evidence for the coupling of transfected human brain A1 adenosine receptors in CHO-K1 cells to mobilisation of [Ca2+]i via a pertussis toxin-sensitive G protein.
Full text
PDF
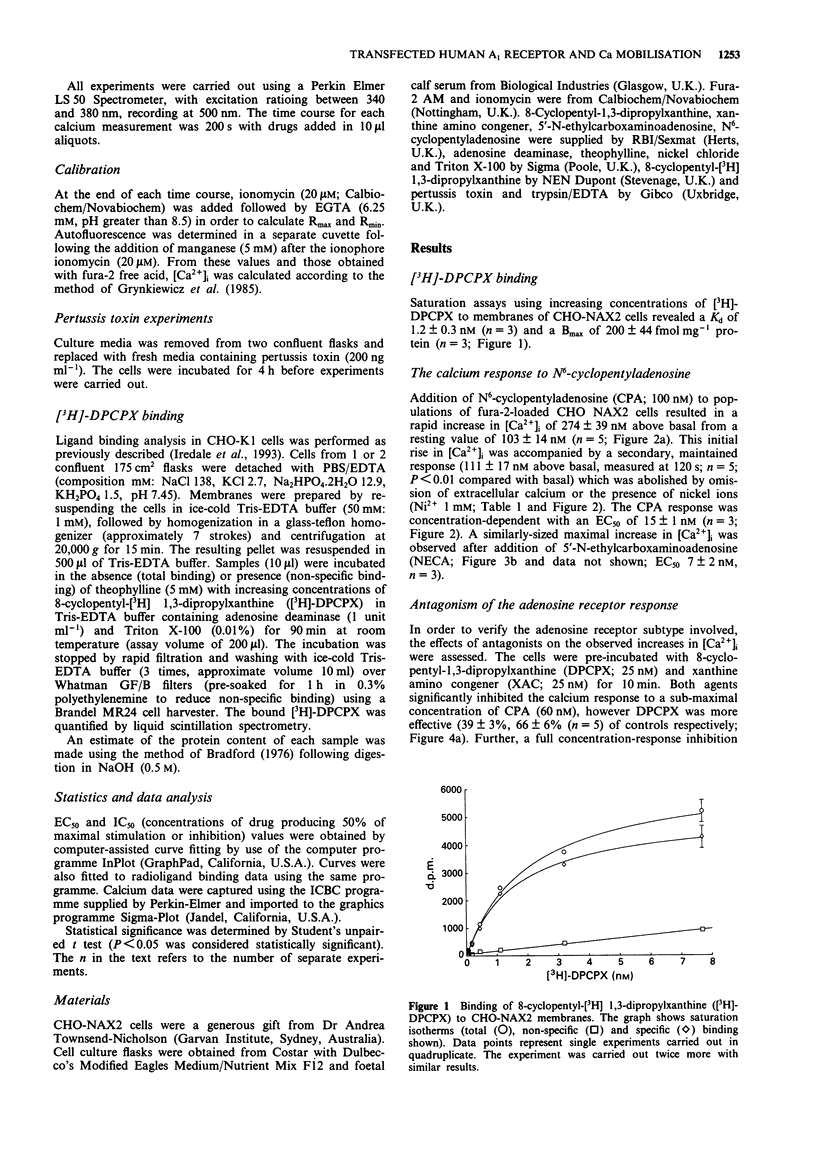
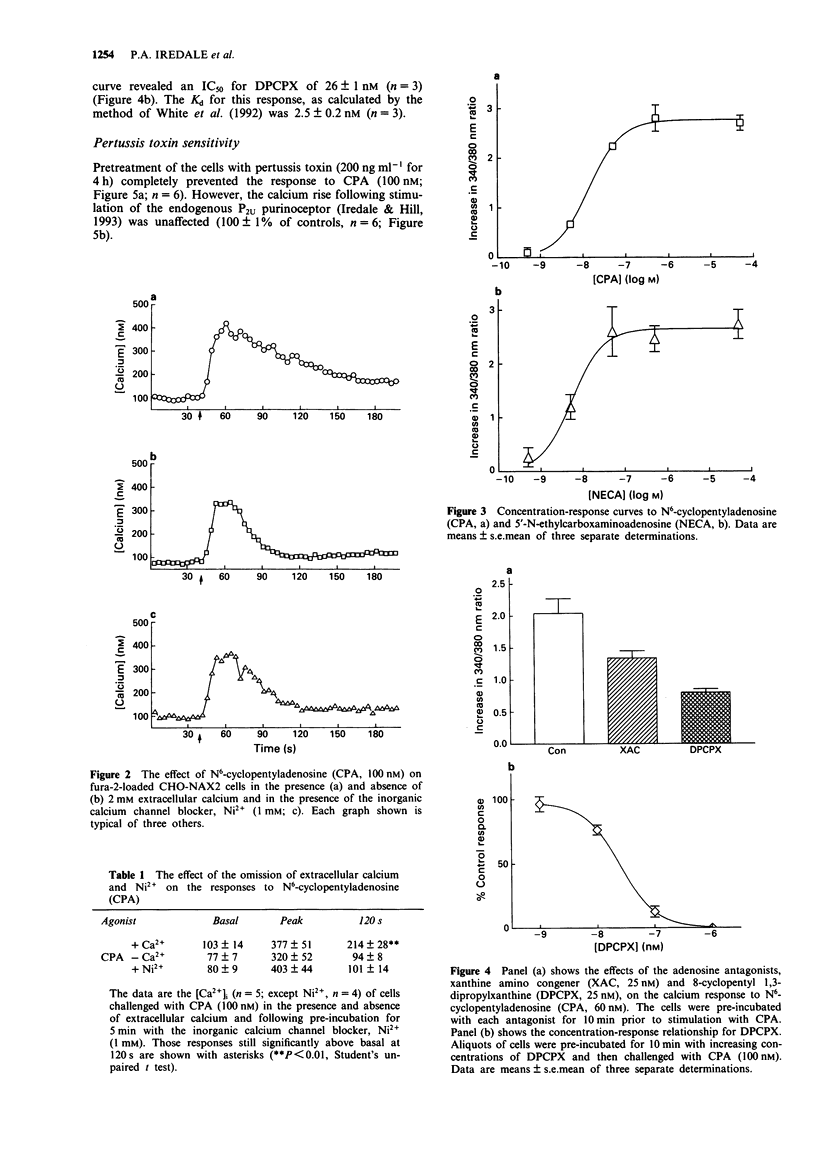
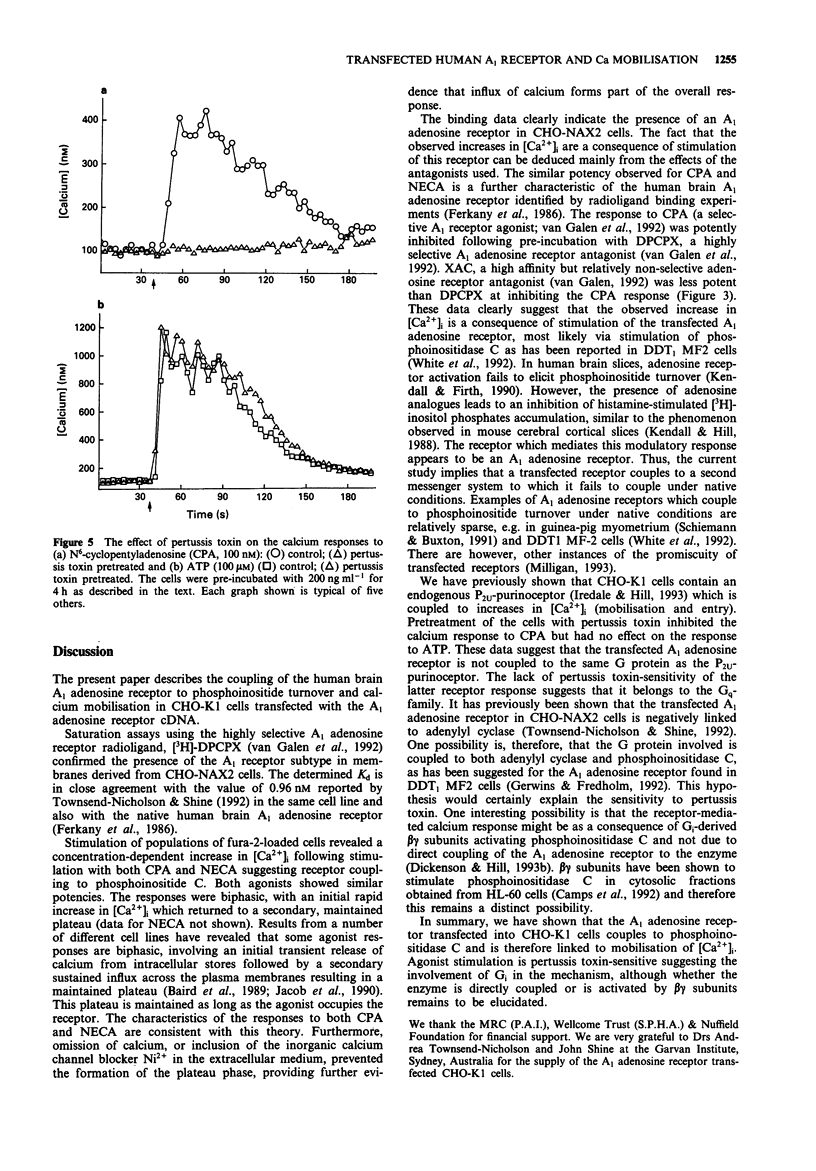

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. G., Lambert D. G., McBain J., Nahorski S. R. Muscarinic receptors coupled to phosphoinositide hydrolysis and elevated cytosolic calcium in a human neuroblastoma cell line SK-N-SH. Br J Pharmacol. 1989 Dec;98(4):1328–1334. doi: 10.1111/j.1476-5381.1989.tb12681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hartman J. D., Hays S. J., Huang C. C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Camps M., Hou C., Sidiropoulos D., Stock J. B., Jakobs K. H., Gierschik P. Stimulation of phospholipase C by guanine-nucleotide-binding protein beta gamma subunits. Eur J Biochem. 1992 Jun 15;206(3):821–831. doi: 10.1111/j.1432-1033.1992.tb16990.x. [DOI] [PubMed] [Google Scholar]
- Carruthers A. M., Fozard J. R. Adenosine A3 receptors: two into one won't go. Trends Pharmacol Sci. 1993 Aug;14(8):290–291. doi: 10.1016/0165-6147(93)90042-I. [DOI] [PubMed] [Google Scholar]
- Chern Y., King K., Lai H. L., Lai H. T. Molecular cloning of a novel adenosine receptor gene from rat brain. Biochem Biophys Res Commun. 1992 May 29;185(1):304–309. doi: 10.1016/s0006-291x(05)90000-4. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Butts-Lamb P., Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983 Mar;3(1):69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Thompson R. D., Kusachi S., Bugni W. J., Olsson R. A. Structure-activity relationships for N6-substituted adenosines at a brain A1-adenosine receptor with a comparison to an A2-adenosine receptor regulating coronary blood flow. Biochem Pharmacol. 1986 Aug 1;35(15):2467–2481. doi: 10.1016/0006-2952(86)90042-0. [DOI] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Adenosine A1-receptor stimulated increases in intracellular calcium in the smooth muscle cell line, DDT1MF-2. Br J Pharmacol. 1993 Jan;108(1):85–92. doi: 10.1111/j.1476-5381.1993.tb13444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Intracellular cross-talk between receptors coupled to phospholipase C via pertussis toxin-sensitive and insensitive G-proteins in DDT1MF-2 cells. Br J Pharmacol. 1993 Jul;109(3):719–724. doi: 10.1111/j.1476-5381.1993.tb13633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong T. J., Pierce K. D., Selbie L. A., Shine J. Molecular characterization of a human brain adenosine A2 receptor. Brain Res Mol Brain Res. 1992 Sep;15(1-2):62–66. doi: 10.1016/0169-328x(92)90152-2. [DOI] [PubMed] [Google Scholar]
- Gerwins P., Fredholm B. B. ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J Biol Chem. 1992 Aug 15;267(23):16081–16087. [PubMed] [Google Scholar]
- Gerwins P., Nordstedt C., Fredholm B. B. Characterization of adenosine A1 receptors in intact DDT1 MF-2 smooth muscle cells. Mol Pharmacol. 1990 Nov;38(5):660–666. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gurden M. F., Coates J., Ellis F., Evans B., Foster M., Hornby E., Kennedy I., Martin D. P., Strong P., Vardey C. J. Functional characterization of three adenosine receptor types. Br J Pharmacol. 1993 Jul;109(3):693–698. doi: 10.1111/j.1476-5381.1993.tb13629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale P. A., Martin K. F., Hill S. J., Kendall D. A. Agonist-induced changes in [Ca2+]i in N1E-115 cells: differential effects of bradykinin and carbachol. Eur J Pharmacol. 1992 Jun 5;226(2):163–168. doi: 10.1016/0922-4106(92)90178-x. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., van Galen P. J., Williams M. Adenosine receptors: pharmacology, structure-activity relationships, and therapeutic potential. J Med Chem. 1992 Feb 7;35(3):407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Firth J. L. Inositol phospholipid hydrolysis in human brain; adenosine inhibition of the response to histamine. Br J Pharmacol. 1990 May;100(1):37–40. doi: 10.1111/j.1476-5381.1990.tb12048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Hill S. J. Adenosine inhibition of histamine-stimulated inositol phospholipid hydrolysis in mouse cerebral cortex. J Neurochem. 1988 Feb;50(2):497–502. doi: 10.1111/j.1471-4159.1988.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Libert F., Schiffmann S. N., Lefort A., Parmentier M., Gérard C., Dumont J. E., Vanderhaeghen J. J., Vassart G. The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J. 1991 Jul;10(7):1677–1682. doi: 10.1002/j.1460-2075.1991.tb07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenhaut C., Van Sande J., Libert F., Abramowicz M., Parmentier M., Vanderhaegen J. J., Dumont J. E., Vassart G., Schiffmann S. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- Mahan L. C., McVittie L. D., Smyk-Randall E. M., Nakata H., Monsma F. J., Jr, Gerfen C. R., Sibley D. R. Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol. 1991 Jul;40(1):1–7. [PubMed] [Google Scholar]
- Milligan G. Mechanisms of multifunctional signalling by G protein-linked receptors. Trends Pharmacol Sci. 1993 Jun;14(6):239–244. doi: 10.1016/0165-6147(93)90019-g. [DOI] [PubMed] [Google Scholar]
- Picking W. D. Interaction of pyrene-labeled monosialoganglioside GM1 micelles with cholera toxin. Biochem Biophys Res Commun. 1993 Sep 30;195(3):1153–1158. doi: 10.1006/bbrc.1993.2165. [DOI] [PubMed] [Google Scholar]
- Pierce K. D., Furlong T. J., Selbie L. A., Shine J. Molecular cloning and expression of an adenosine A2b receptor from human brain. Biochem Biophys Res Commun. 1992 Aug 31;187(1):86–93. doi: 10.1016/s0006-291x(05)81462-7. [DOI] [PubMed] [Google Scholar]
- Schiemann W. P., Buxton I. L. Adenosine A1-receptor coupling to phosphoinositide metabolism in pregnant guinea pig myometrium. Am J Physiol. 1991 Nov;261(5 Pt 1):E665–E672. doi: 10.1152/ajpendo.1991.261.5.E665. [DOI] [PubMed] [Google Scholar]
- Stehle J. H., Rivkees S. A., Lee J. J., Weaver D. R., Deeds J. D., Reppert S. M. Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol. 1992 Mar;6(3):384–393. doi: 10.1210/mend.6.3.1584214. [DOI] [PubMed] [Google Scholar]
- Townsend-Nicholson A., Shine J. Molecular cloning and characterisation of a human brain A1 adenosine receptor cDNA. Brain Res Mol Brain Res. 1992 Dec;16(3-4):365–370. doi: 10.1016/0169-328x(92)90248-a. [DOI] [PubMed] [Google Scholar]
- White T. E., Dickenson J. M., Alexander S. P., Hill S. J. Adenosine A1-receptor stimulation of inositol phospholipid hydrolysis and calcium mobilisation in DDT1 MF-2 cells. Br J Pharmacol. 1992 May;106(1):215–221. doi: 10.1111/j.1476-5381.1992.tb14317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. Y., Li C., Olah M. E., Johnson R. A., Stiles G. L., Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- van Galen P. J., Stiles G. L., Michaels G., Jacobson K. A. Adenosine A1 and A2 receptors: structure--function relationships. Med Res Rev. 1992 Sep;12(5):423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]


