Abstract
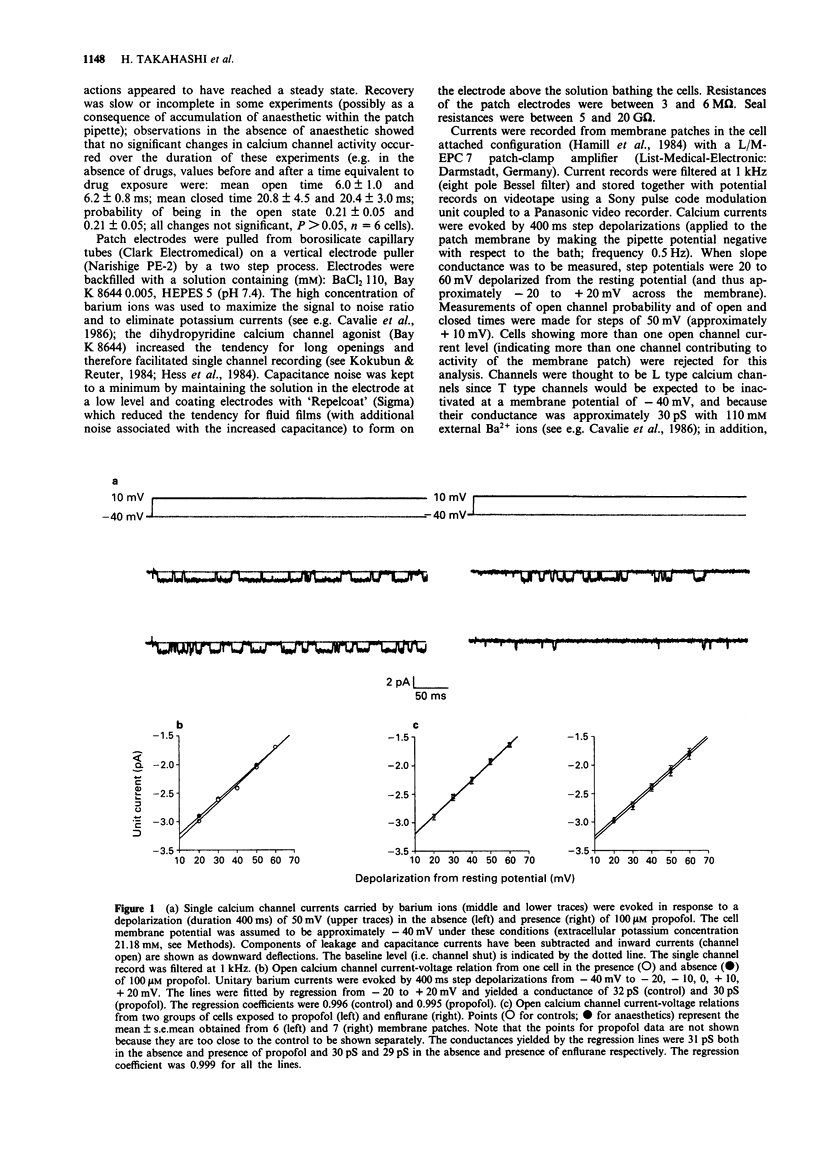
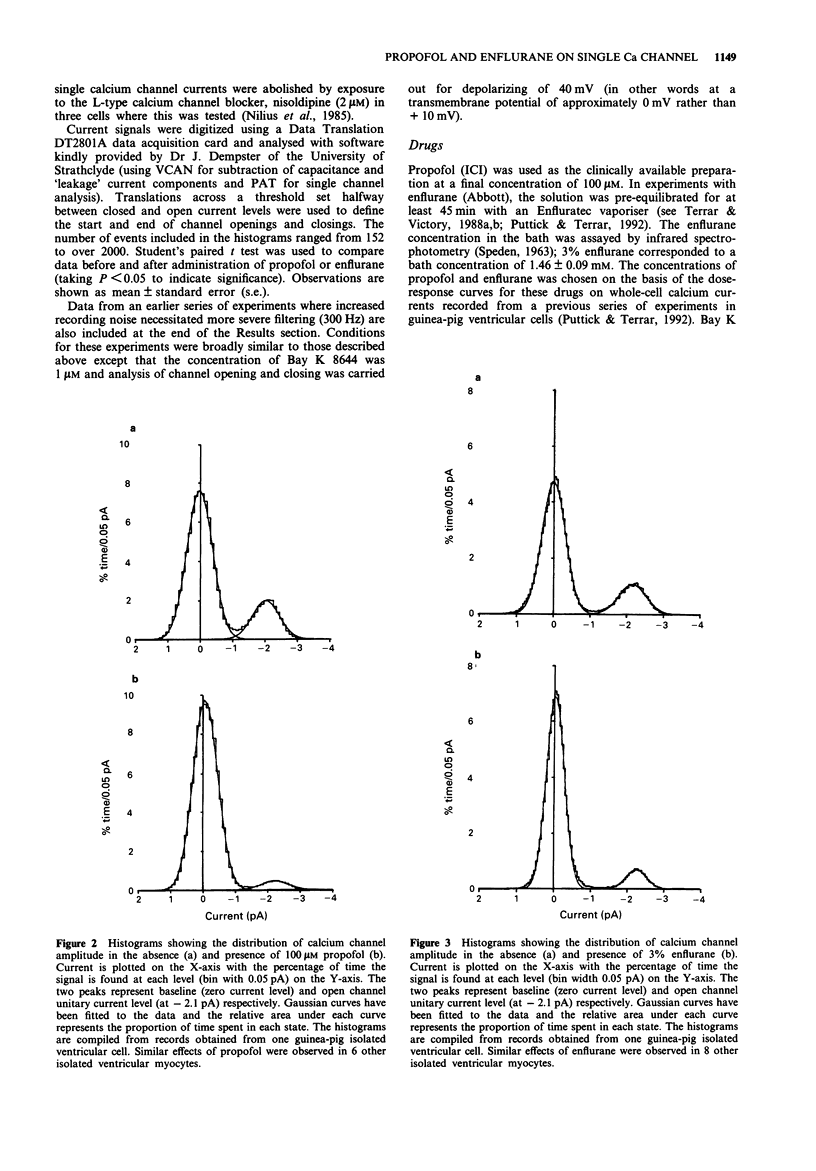
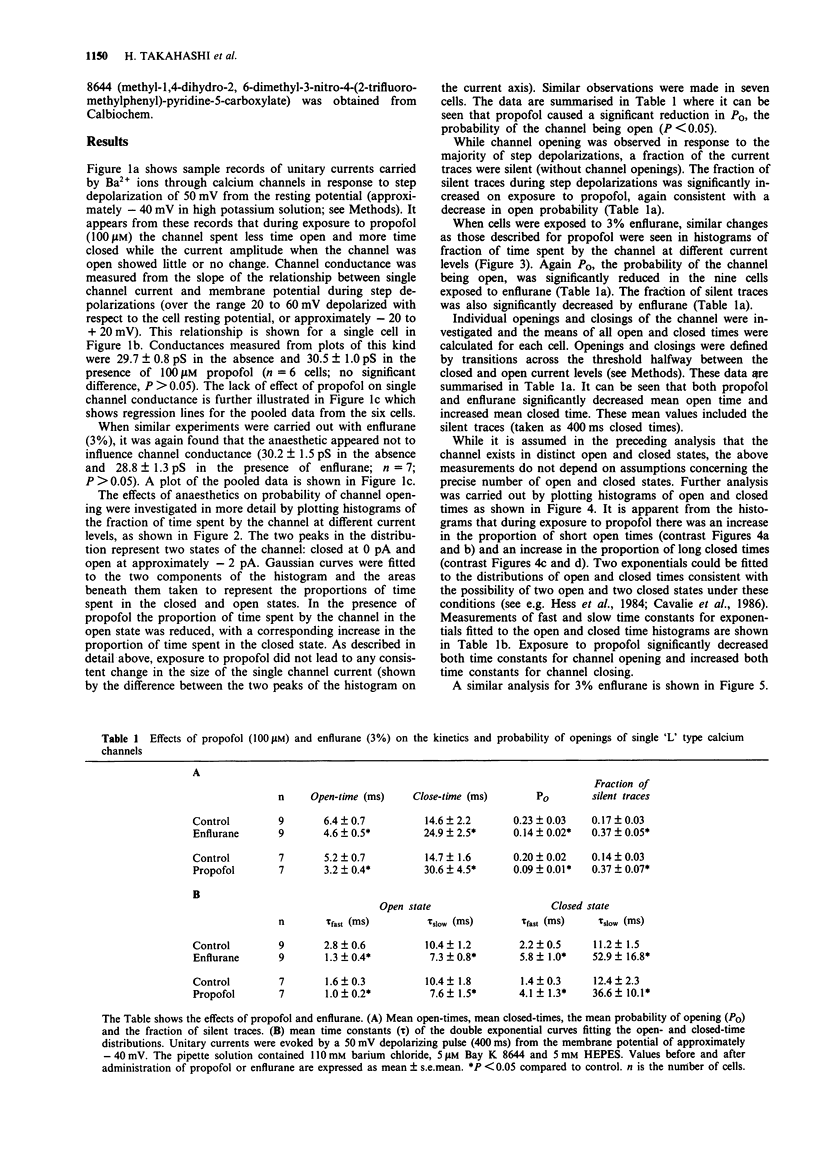
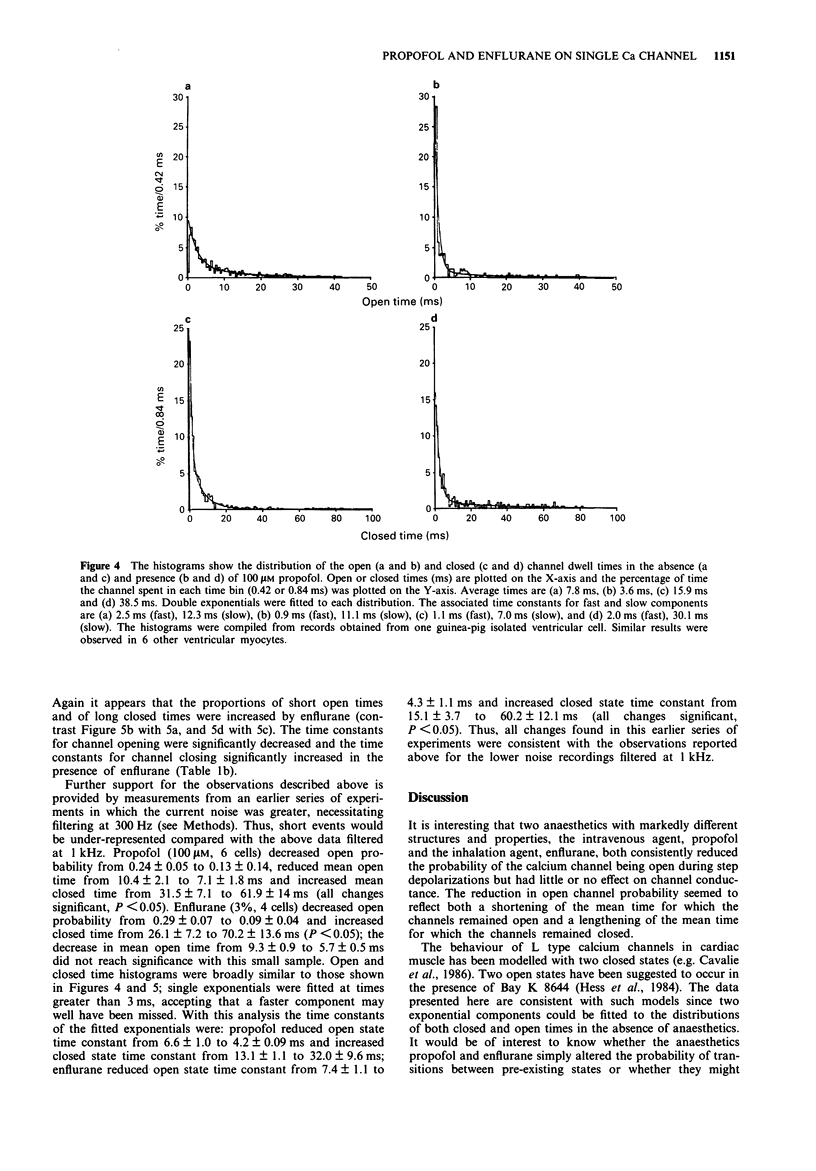
1. The effects of the anaesthetics, propofol (100 microM) and enflurane (3%, 1.46 mM), on single L type calcium channel currents were investigated in single myocytes isolated from guinea-pig ventricles. Channel activity was recorded from membrane patches by use of the 'cell-attached' patch-clamp technique (pipette solution containing 110 mM BaCl2, 5 microM Bay K 8644, 5 microM HEPES, pH 7.4; temperature 36 degrees C). 2. Channel conductance was calculated from the slope of the relationship between single channel current and membrane potential during step depolarizations to activate the channel over a range of approximately -20 to +20 mV. Neither propofol (6 cells) nor enflurane (7 cells) caused any significant reduction in channel conductance. 3. Both propofol (7 cells) and enflurane (9 cells) decreased the probability of the channel being open during depolarizations to +10 mV (measured from histograms of the fraction of time spent by the channel at different current levels, taking areas under the Gaussian curves fitted to the open and closed components of the distributions to represent the proportion of time spent in the two states). 4. A fraction of the current traces showed no detectable channel openings in response to step depolarizations to +10 mV. Both propofol and enflurane significantly increased the fraction of silent traces. 5. Transitions across a threshold halfway between the open and closed levels were used to define periods spent in the open and closed states. Both propofol (7 cells) and enflurane (9 cells) reduced the mean open times and increased the mean closed times of the calcium channel.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosnjak Z. J., Supan F. D., Rusch N. J. The effects of halothane, enflurane, and isoflurane on calcium current in isolated canine ventricular cells. Anesthesiology. 1991 Feb;74(2):340–345. doi: 10.1097/00000542-199102000-00022. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hirota K., Ito Y., Masuda A., Momose Y. Effects of halothane on membrane ionic currents in guinea pig atrial and ventricular myocytes. Acta Anaesthesiol Scand. 1989 Apr;33(3):239–244. doi: 10.1111/j.1399-6576.1989.tb02898.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto Y., Yatani A., Arimura H., Yoshitake J. Reduction of the slow inward current of isolated rat ventricular cells by thiamylal and halothane. Acta Anaesthesiol Scand. 1985 Aug;29(6):583–586. doi: 10.1111/j.1399-6576.1985.tb02258.x. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda A. E., Brown A. M. Nonmodal gating of cardiac calcium channels as revealed by dihydropyridines. J Gen Physiol. 1989 Jun;93(6):1243–1273. doi: 10.1085/jgp.93.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Niggli E., Rüdisüli A., Maurer P., Weingart R. Effects of general anesthetics on current flow across membranes in guinea pig myocytes. Am J Physiol. 1989 Feb;256(2 Pt 1):C273–C281. doi: 10.1152/ajpcell.1989.256.2.C273. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttick R. M., Terrar D. A. Effects of propofol and enflurane on action potentials, membrane currents and contraction of guinea-pig isolated ventricular myocytes. Br J Pharmacol. 1992 Oct;107(2):559–565. doi: 10.1111/j.1476-5381.1992.tb12783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrar D. A., Victory J. G. Effects of halothane on membrane currents associated with contraction in single myocytes isolated from guinea-pig ventricle. Br J Pharmacol. 1988 Jun;94(2):500–508. doi: 10.1111/j.1476-5381.1988.tb11553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrar D. A., Victory J. G. Isoflurane depresses membrane currents associated with contraction in myocytes isolated from guinea-pig ventricle. Anesthesiology. 1988 Nov;69(5):742–749. doi: 10.1097/00000542-198811000-00017. [DOI] [PubMed] [Google Scholar]


