Abstract
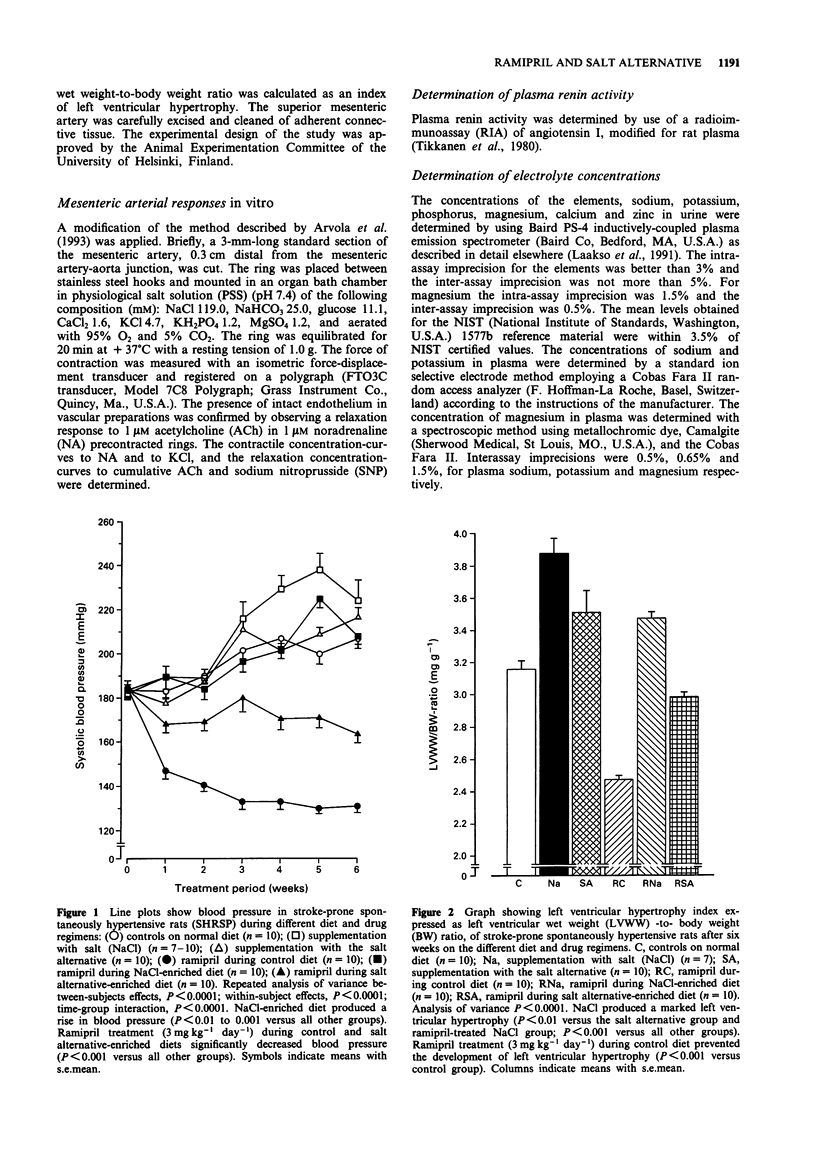
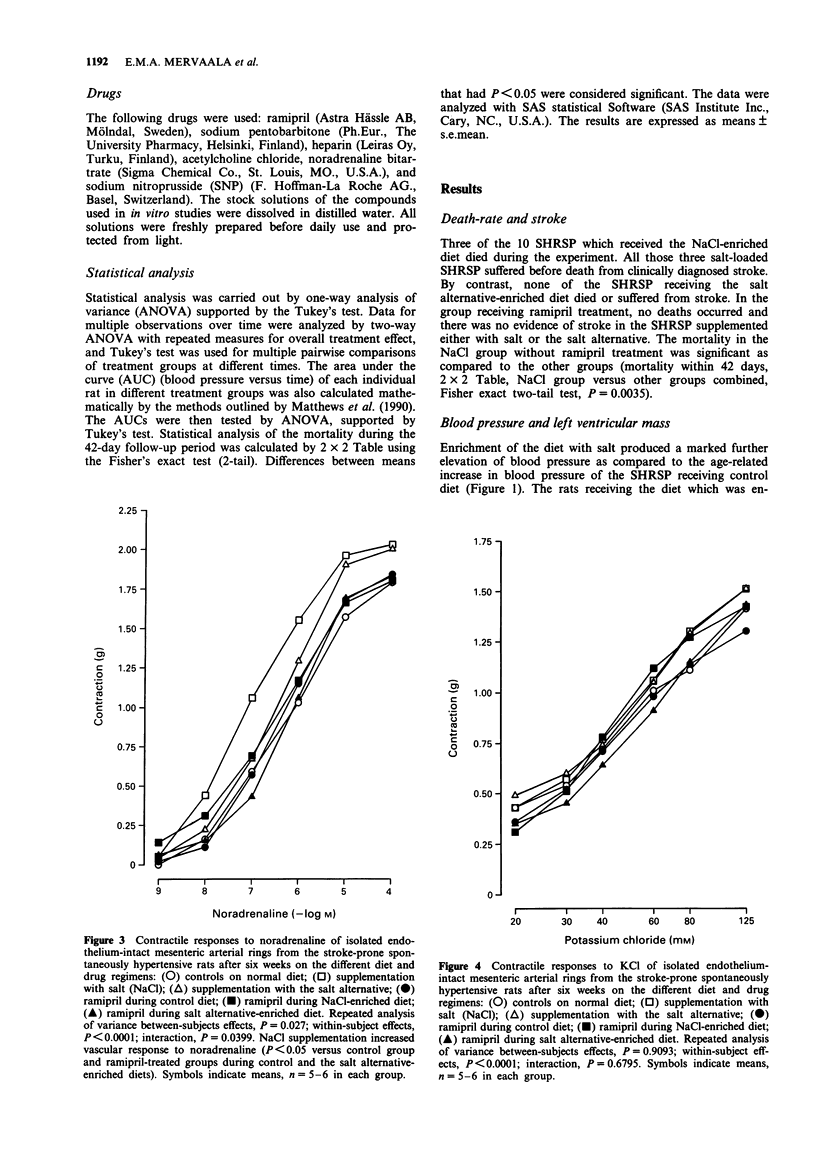
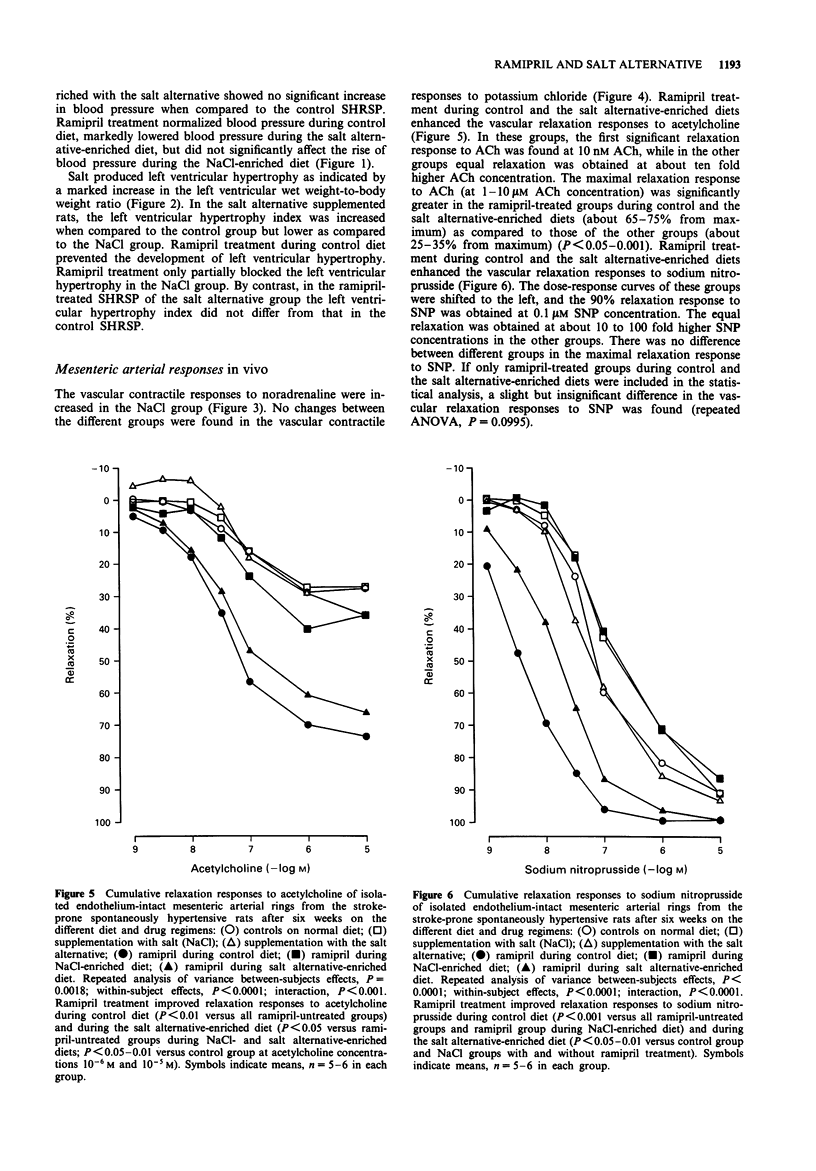
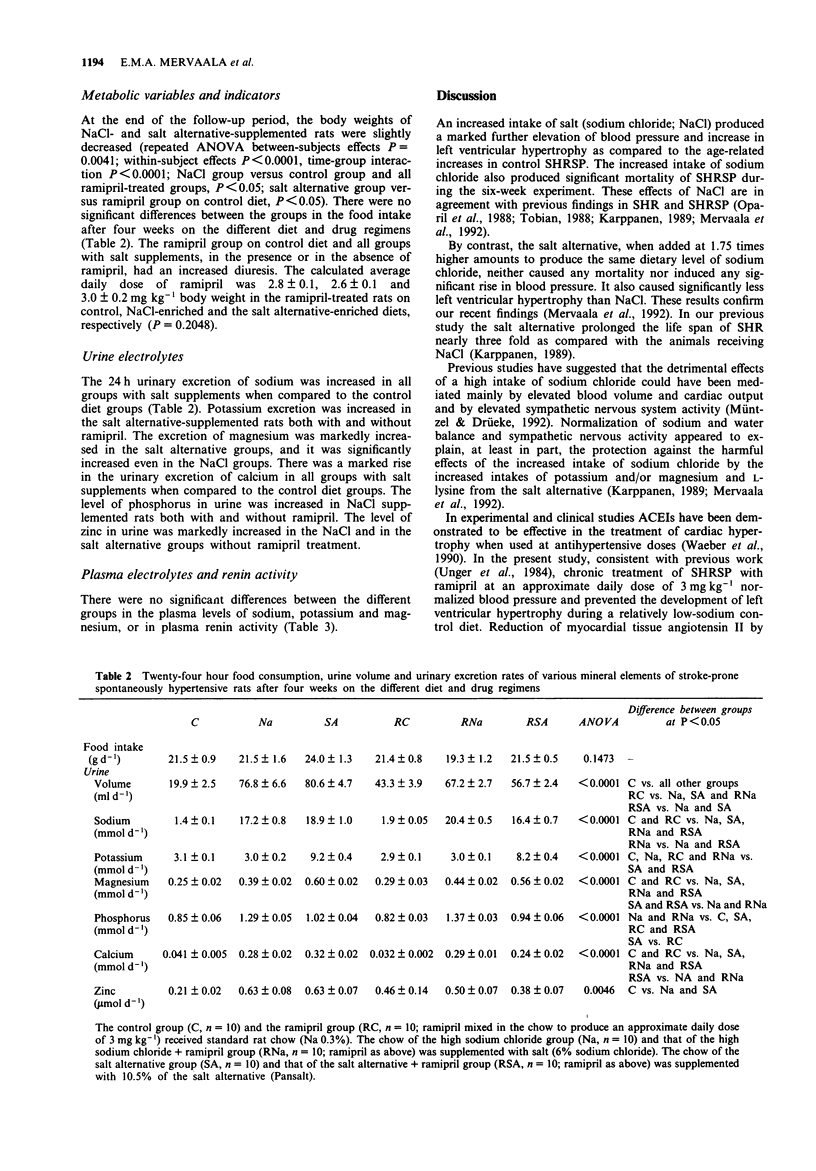
1. The influence of salt (sodium chloride; NaCl) (an additional 6% in the diet) and that of a novel sodium-reduced, potassium-, magnesium-, and L-lysine-enriched salt alternative on the cardiovascular effects of ramipril was studied in stroke-prone spontaneously hypertensive rats in a 6-week study. The intake of sodium chloride was adjusted to the same level by adding the salt alternative at a 1.75 times higher amount than regular salt. 2. Salt produced a marked rise in blood pressure and induced cardiac hypertrophy and significant mortality, while the salt alternative neither increased blood pressure nor caused any mortality and produced less cardiac hypertrophy than salt. 3. Ramipril treatment at a daily dose of 3 mg kg-1 normalized blood pressure and prevented the development of cardiac hypertrophy of rats on control diet. These effects of ramipril were blocked by the addition of salt but were only slightly attenuated by the addition of the salt alternative. The mortality in the salt group was prevented by ramipril. 4. Responses of mesenteric arterial rings in vitro were examined at the end of the study. Salt, but not the salt alternative, increased vascular contractile responses to noradrenaline. Ramipril treatment improved the arterial relaxation responses to acetylcholine and to sodium nitroprusside. The vascular relaxation enhancing effect of ramipril was blocked by salt but only slightly attenuated by the salt alternative. 5. Ramipril treatment did not significantly increase plasma renin activity in the presence or in the absence of salt supplementation. The salt alternative did not cause hyperkalaemia, either alone or in combination with ramipril treatment.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Altura B. T. Cardiovascular risk factors and magnesium: relationships to atherosclerosis, ischemic heart disease and hypertension. Magnes Trace Elem. 1991;10(2-4):182–192. [PubMed] [Google Scholar]
- Arvola P., Ruskoaho H., Wuorela H., Pekki A., Vapaatalo H., Pörsti I. Quinapril treatment and arterial smooth muscle responses in spontaneously hypertensive rats. Br J Pharmacol. 1993 Apr;108(4):980–990. doi: 10.1111/j.1476-5381.1993.tb13495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilla C. G., Janicki J. S., Weber K. T. Cardioreparative effects of lisinopril in rats with genetic hypertension and left ventricular hypertrophy. Circulation. 1991 May;83(5):1771–1779. doi: 10.1161/01.cir.83.5.1771. [DOI] [PubMed] [Google Scholar]
- Cappuccio F. P., MacGregor G. A. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens. 1991 May;9(5):465–473. doi: 10.1097/00004872-199105000-00011. [DOI] [PubMed] [Google Scholar]
- Childs T. J., Adams M. A., Mak A. S. Regression of cardiac hypertrophy in spontaneously hypertensive rats by enalapril and the expression of contractile proteins. Hypertension. 1990 Dec;16(6):662–668. doi: 10.1161/01.hyp.16.6.662. [DOI] [PubMed] [Google Scholar]
- Clozel J. P., Kuhn H., Hefti F. Effects of chronic ACE inhibition on cardiac hypertrophy and coronary vascular reserve in spontaneously hypertensive rats with developed hypertension. J Hypertens. 1989 Apr;7(4):267–275. [PubMed] [Google Scholar]
- Clozel M., Kuhn H., Hefti F. Effects of angiotensin converting enzyme inhibitors and of hydralazine on endothelial function in hypertensive rats. Hypertension. 1990 Nov;16(5):532–540. doi: 10.1161/01.hyp.16.5.532. [DOI] [PubMed] [Google Scholar]
- Frost C. D., Law M. R., Wald N. J. By how much does dietary salt reduction lower blood pressure? II--Analysis of observational data within populations. BMJ. 1991 Apr 6;302(6780):815–818. doi: 10.1136/bmj.302.6780.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C., Karlberg B. E. Plasma concentrations of angiotensin II and aldosterone during acute left ventricular failure in the dog. Effect of converting enzyme inhibition. Res Exp Med (Berl) 1986;186(5):387–395. doi: 10.1007/BF01852104. [DOI] [PubMed] [Google Scholar]
- Jula A., Rönnemaa T., Rastas M., Karvetti R. L., Mäki J. Long-term nopharmacological treatment for mild to moderate hypertension. J Intern Med. 1990 Jun;227(6):413–421. doi: 10.1111/j.1365-2796.1990.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Karppanen H. New oral salt in treatment of high blood pressure. Magnesium. 1989;8(5-6):274–287. [PubMed] [Google Scholar]
- Kato H., Suzuki H., Tajima S., Ogata Y., Tominaga T., Sato A., Saruta T. Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J Hypertens. 1991 Jan;9(1):17–22. [PubMed] [Google Scholar]
- Konishi M., Su C. Role of endothelium in dilator responses of spontaneously hypertensive rat arteries. Hypertension. 1983 Nov-Dec;5(6):881–886. doi: 10.1161/01.hyp.5.6.881. [DOI] [PubMed] [Google Scholar]
- Law M. R., Frost C. D., Wald N. J. By how much does dietary salt reduction lower blood pressure? I--Analysis of observational data among populations. BMJ. 1991 Apr 6;302(6780):811–815. doi: 10.1136/bmj.302.6780.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. R., Frost C. D., Wald N. J. By how much does dietary salt reduction lower blood pressure? III--Analysis of data from trials of salt reduction. BMJ. 1991 Apr 6;302(6780):819–824. doi: 10.1136/bmj.302.6780.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L., Lithell H., Pollare T., Ljunghall S. Blood pressure response during long-term treatment with magnesium is dependent on magnesium status. A double-blind, placebo-controlled study in essential hypertension and in subjects with high-normal blood pressure. Am J Hypertens. 1991 Aug;4(8):674–679. doi: 10.1093/ajh/4.8.674. [DOI] [PubMed] [Google Scholar]
- Lindpaintner K., Ganten D. The cardiac renin-angiotensin system. An appraisal of present experimental and clinical evidence. Circ Res. 1991 Apr;68(4):905–921. doi: 10.1161/01.res.68.4.905. [DOI] [PubMed] [Google Scholar]
- Linz W., Schaper J., Wiemer G., Albus U., Schölkens B. A. Ramipril prevents left ventricular hypertrophy with myocardial fibrosis without blood pressure reduction: a one year study in rats. Br J Pharmacol. 1992 Dec;107(4):970–975. doi: 10.1111/j.1476-5381.1992.tb13393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher T. F., Raij L., Vanhoutte P. M. Endothelium-dependent vascular responses in normotensive and hypertensive Dahl rats. Hypertension. 1987 Feb;9(2):157–163. doi: 10.1161/01.hyp.9.2.157. [DOI] [PubMed] [Google Scholar]
- Matthews J. N., Altman D. G., Campbell M. J., Royston P. Analysis of serial measurements in medical research. BMJ. 1990 Jan 27;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervaala E. M., Himberg J. J., Laakso J., Tuomainen P., Karppanen H. Beneficial effects of a potassium- and magnesium-enriched salt alternative. Hypertension. 1992 Jun;19(6 Pt 1):535–540. doi: 10.1161/01.hyp.19.6.535. [DOI] [PubMed] [Google Scholar]
- Muntzel M., Drüeke T. A comprehensive review of the salt and blood pressure relationship. Am J Hypertens. 1992 Apr;5(4 Pt 1):1S–42S. doi: 10.1093/ajh/5.4s.1s. [DOI] [PubMed] [Google Scholar]
- Nagano M., Higaki J., Mikami H., Nakamaru M., Higashimori K., Katahira K., Tabuchi Y., Moriguchi A., Nakamura F., Ogihara T. Converting enzyme inhibitors regressed cardiac hypertrophy and reduced tissue angiotensin II in spontaneously hypertensive rats. J Hypertens. 1991 Jul;9(7):595–599. doi: 10.1097/00004872-199107000-00003. [DOI] [PubMed] [Google Scholar]
- Oparil S., Meng Q. C., Chen Y. F., Yang R. H., Jin H. K., Wyss J. M. Genetic basis of NaCl-sensitive hypertension. J Cardiovasc Pharmacol. 1988;12 (Suppl 3):S56–S69. [PubMed] [Google Scholar]
- Pfeffer J. M., Pfeffer M. A., Mirsky I., Braunwald E. Regression of left ventricular hypertrophy and prevention of left ventricular dysfunction by captopril in the spontaneously hypertensive rat. Proc Natl Acad Sci U S A. 1982 May;79(10):3310–3314. doi: 10.1073/pnas.79.10.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H., Dzau V. J., Tang S. S., Hirsch A. T., Apstein C. S., Lorell B. H. Increased rat cardiac angiotensin converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. Effects on coronary resistance, contractility, and relaxation. J Clin Invest. 1990 Dec;86(6):1913–1920. doi: 10.1172/JCI114924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren A., Edvinsson L., Fallgren B. Magnesium deficiency in coronary artery disease and cardiac arrhythmias. J Intern Med. 1989 Oct;226(4):213–222. doi: 10.1111/j.1365-2796.1989.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Stier C. T., Jr, Benter I. F., Ahmad S., Zuo H. L., Selig N., Roethel S., Levine S., Itskovitz H. D. Enalapril prevents stroke and kidney dysfunction in salt-loaded stroke-prone spontaneously hypertensive rats. Hypertension. 1989 Feb;13(2):115–121. doi: 10.1161/01.hyp.13.2.115. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Tobian L., Ganguli M. C. High potassium diets protect against dysfunction of endothelial cells in stroke-prone spontaneously hypertensive rats. Hypertension. 1988 Jun;11(6 Pt 2):579–585. doi: 10.1161/01.hyp.11.6.579. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Halpern W. Endothelium-dependent and endothelium-independent vasodilation in resistance arteries from hypertensive rats. Hypertension. 1988 May;11(5):440–444. doi: 10.1161/01.hyp.11.5.440. [DOI] [PubMed] [Google Scholar]
- Tikkanen I., Fyhrquist F., Puutula-Räsänen L. Enzyme inhibitors for renin assay in rat plasma. Clin Sci (Lond) 1980 Nov;59(5):381–383. doi: 10.1042/cs0590381. [DOI] [PubMed] [Google Scholar]
- Unger T., Fleck T., Ganten D., Lang R. E., Rettig F. 2-[N-[(S)-1-ethoxycarbonyl-3-phenylpropyl]-L-alanyl]-(1S,3S,5S)-2- azabicyclo[3.3.0]octane-3-carboxylic acid (Hoe 498): antihypertensive action and persistent inhibition of tissue converting enzyme activity in spontaneously hypertensive rats. Arzneimittelforschung. 1984;34(10B):1426–1430. [PubMed] [Google Scholar]
- Wallach S., Verch R. L. Tissue magnesium in spontaneously hypertensive rats. Magnesium. 1986;5(1):33–38. [PubMed] [Google Scholar]
- Wiemer G., Schölkens B. A., Becker R. H., Busse R. Ramiprilat enhances endothelial autacoid formation by inhibiting breakdown of endothelium-derived bradykinin. Hypertension. 1991 Oct;18(4):558–563. doi: 10.1161/01.hyp.18.4.558. [DOI] [PubMed] [Google Scholar]
- Yamori Y., Horie R., Akiguchi I., Kihara M., Nara Y., Lovenberg W. Symptomatological classification in the development of stroke in stroke-prone spontaneously hypertensive rats. Jpn Circ J. 1982 Mar;46(3):274–283. doi: 10.1253/jcj.46.274. [DOI] [PubMed] [Google Scholar]
- Young D. B., McCaa R. E., Pan Y. J., Guyton A. C. The natriuretic and hypotensive effects of potassium. Circ Res. 1976 Jun;38(6 Suppl 2):84–89. doi: 10.1161/01.res.38.6.84. [DOI] [PubMed] [Google Scholar]


