Abstract
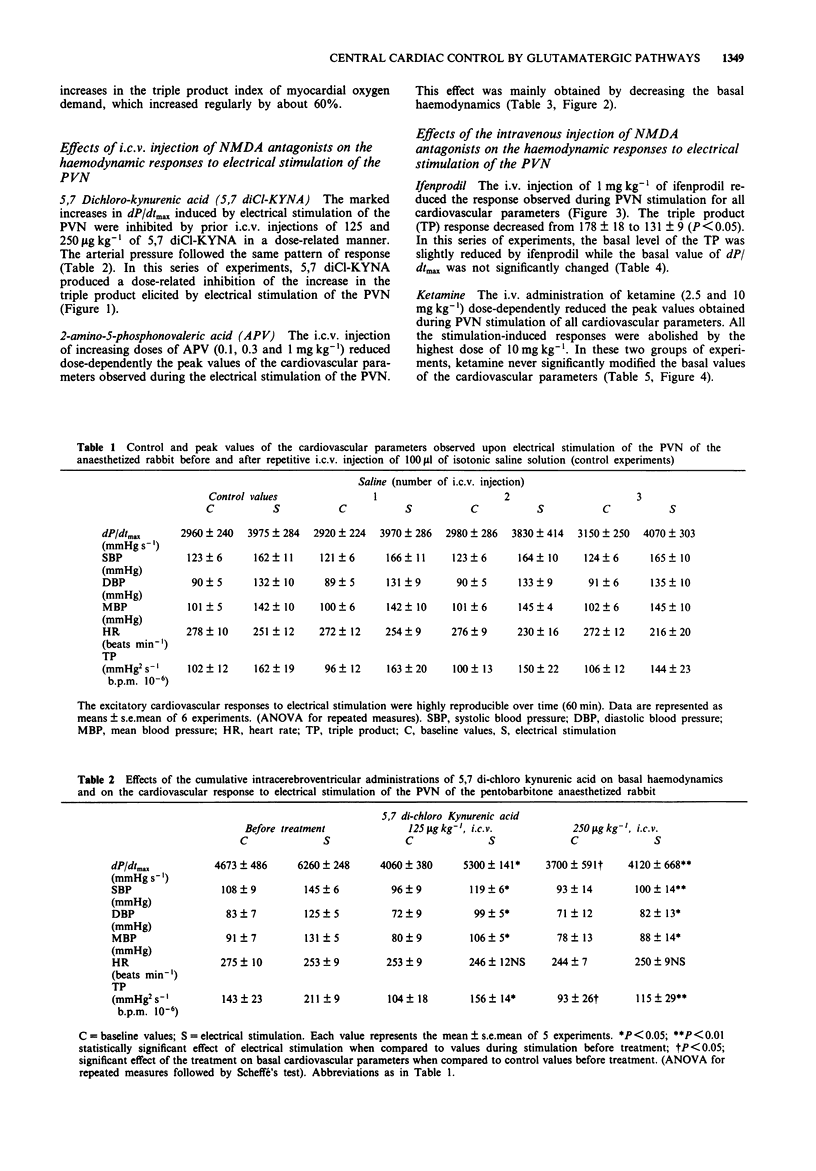
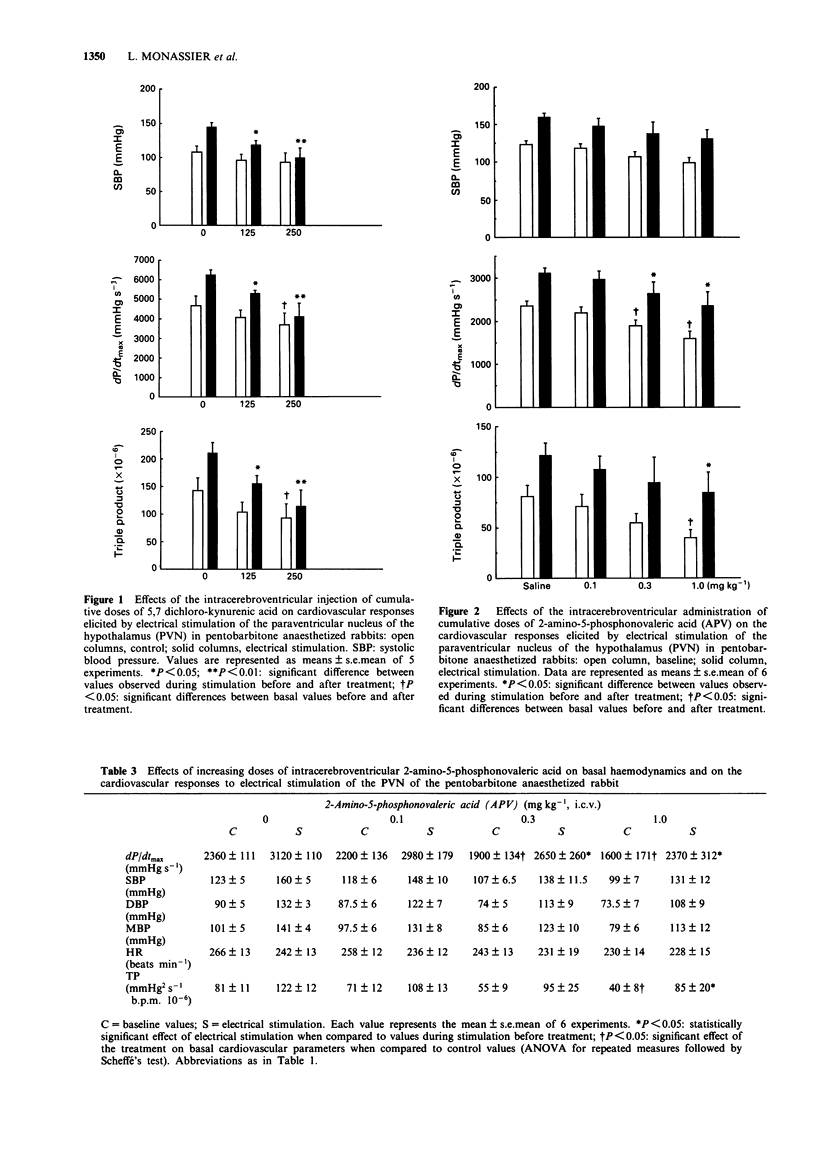
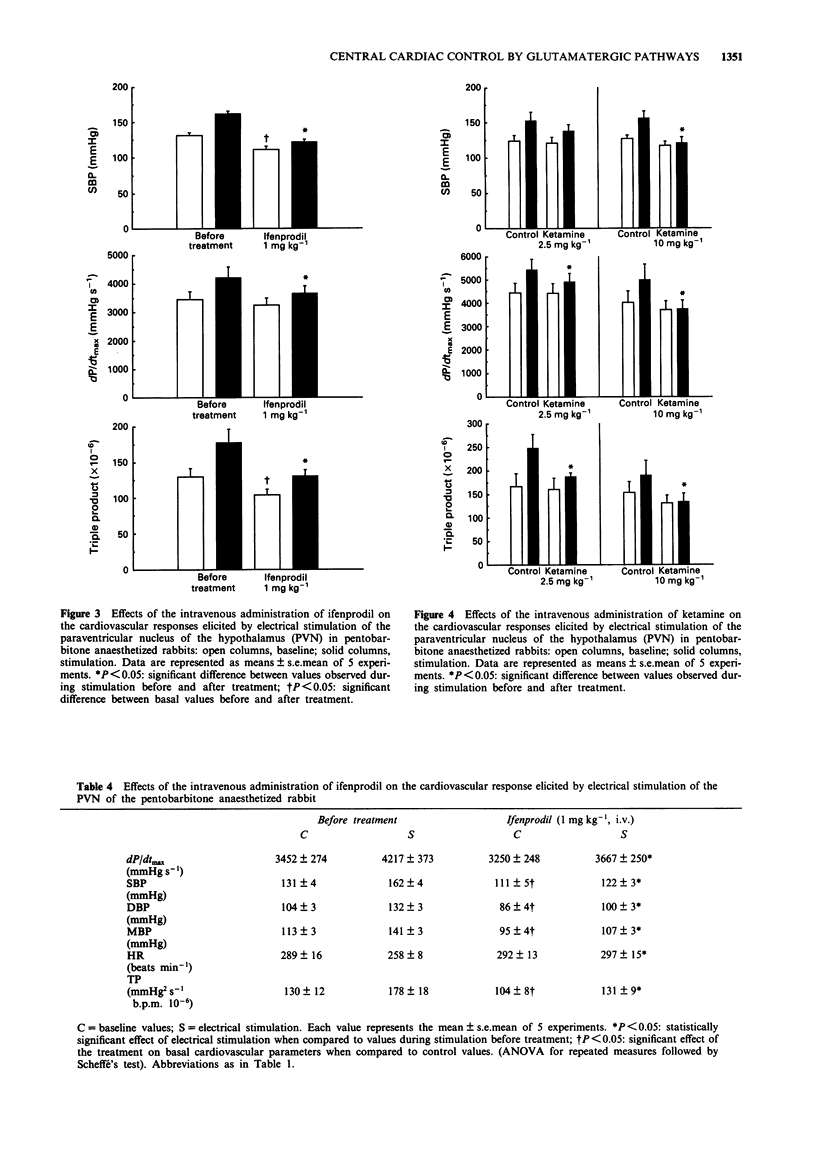
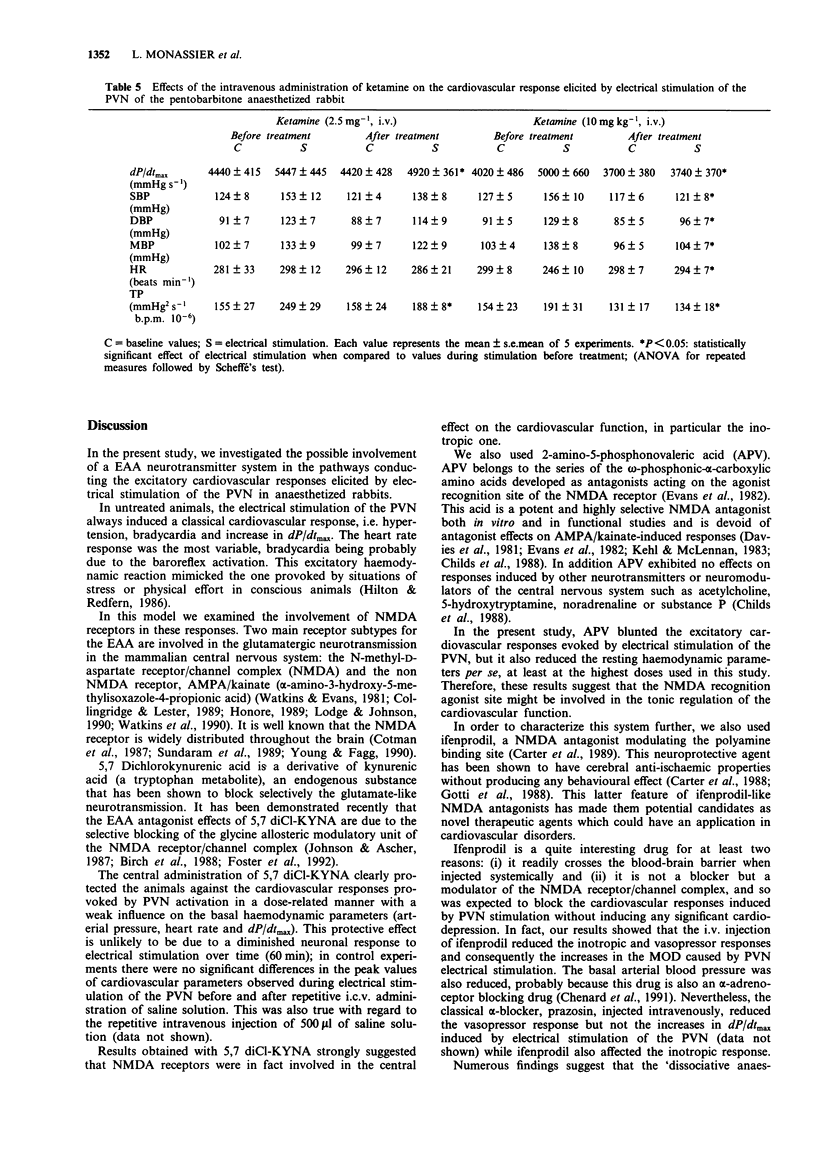
1. The purpose of this study was to investigate further the role of the excitatory amino acid (EAA) system of neurotransmission, particularly of the NMDA receptor, in the central regulation of cardiac function. 2. Electrical stimulation of the paraventricular nucleus of the hypothalamus (PVN) in pentobarbitone anaesthetized rabbits induced a cardiovascular response mainly characterized by a positive inotropic effect, hypertension and a marked increase in the myocardial oxygen demand index. 3. The intracerebroventricular (i.c.v.) or intravenous (i.v.) injection of different EAA antagonists acting on different sites of the NMDA receptor/channel complex dose-dependently blunted the excitatory cardiovascular effects of PVN stimulation. 4. 5,7 Dichlorokynurenic acid was used as a specific glycine site antagonist and 2-amino-5-phosphonovaleric acid was used to block the agonist recognition site; ketamine was used as a channel blocker site antagonist and ifenprodil as a blocker of the polyamine binding site. 5. 5,7 Dichlorokynurenic acid (125 and 250 micrograms kg-1, i.c.v.) virtually abolished the cardiovascular responses, inducing only haemodynamic depression at the highest dose used. 2-Amino-5-phosphonovaleric acid (0.1 to 1.0 mg kg-1, i.c.v.) elicited a reduction of the peak values observed during PVN stimulation which was accompanied by a decrease of the basal cardiovascular parameters. Ketamine (2.5 and 10 mg kg-1) and ifenprodil (1 mg kg-1), injected intravenously, blocked the haemodynamic response induced by PVN stimulation without marked reduction of the basal haemodynamics. 6. It is concluded that glutamate neurotransmission is not only involved in vasomotor tone control but also in the central control of cardiac function and can therefore modulate the myocardial oxygen demand.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baller D., Bretschneider H. J., Hellige G. Validity of myocardial oxygen consumption parameters. Clin Cardiol. 1979 Oct;2(5):317–327. doi: 10.1002/clc.4960020502. [DOI] [PubMed] [Google Scholar]
- Birch P. J., Grossman C. J., Hayes A. G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Sep 1;154(1):85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Carter C., Benavides J., Legendre P., Vincent J. D., Noel F., Thuret F., Lloyd K. G., Arbilla S., Zivkovic B., MacKenzie E. T. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. II. Evidence for N-methyl-D-aspartate receptor antagonist properties. J Pharmacol Exp Ther. 1988 Dec;247(3):1222–1232. [PubMed] [Google Scholar]
- Carter C., Rivy J. P., Scatton B. Ifenprodil and SL 82.0715 are antagonists at the polyamine site of the N-methyl-D-aspartate (NMDA) receptor. Eur J Pharmacol. 1989 May 30;164(3):611–612. doi: 10.1016/0014-2999(89)90275-6. [DOI] [PubMed] [Google Scholar]
- Chalmers J., Pilowsky P. Brainstem and bulbospinal neurotransmitter systems in the control of blood pressure. J Hypertens. 1991 Aug;9(8):675–694. doi: 10.1097/00004872-199108000-00001. [DOI] [PubMed] [Google Scholar]
- Chenard B. L., Shalaby I. A., Koe B. K., Ronau R. T., Butler T. W., Prochniak M. A., Schmidt A. W., Fox C. B. Separation of alpha 1 adrenergic and N-methyl-D-aspartate antagonist activity in a series of ifenprodil compounds. J Med Chem. 1991 Oct;34(10):3085–3090. doi: 10.1021/jm00114a018. [DOI] [PubMed] [Google Scholar]
- Childs A. M., Evans R. H., Watkins J. C. The pharmacological selectivity of three NMDA antagonists. Eur J Pharmacol. 1988 Jan 5;145(1):81–86. doi: 10.1016/0014-2999(88)90352-4. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983 Jan;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Martin D., Millar J. D., Aram J. A., Church J., Lodge D. Differences in results from in vivo and in vitro studies on the use-dependency of N-methylaspartate antagonism by MK-801 and other phencyclidine receptor ligands. Eur J Pharmacol. 1988 Jan 12;145(2):141–151. doi: 10.1016/0014-2999(88)90225-7. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Jones A. W., Smith D. A., Watkins J. C. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982 Jan;75(1):65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Kemp J. A., Leeson P. D., Grimwood S., Donald A. E., Marshall G. R., Priestley T., Smith J. D., Carling R. W. Kynurenic acid analogues with improved affinity and selectivity for the glycine site on the N-methyl-D-aspartate receptor from rat brain. Mol Pharmacol. 1992 May;41(5):914–922. [PubMed] [Google Scholar]
- Gotti B., Duverger D., Bertin J., Carter C., Dupont R., Frost J., Gaudilliere B., MacKenzie E. T., Rousseau J., Scatton B. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. I. Evidence for efficacy in models of focal cerebral ischemia. J Pharmacol Exp Ther. 1988 Dec;247(3):1211–1221. [PubMed] [Google Scholar]
- Guyenet P. G., Filtz T. M., Donaldson S. R. Role of excitatory amino acids in rat vagal and sympathetic baroreflexes. Brain Res. 1987 Mar 31;407(2):272–284. doi: 10.1016/0006-8993(87)91105-x. [DOI] [PubMed] [Google Scholar]
- Halliwell R. F., Peters J. A., Lambert J. J. The mechanism of action and pharmacological specificity of the anticonvulsant NMDA antagonist MK-801: a voltage clamp study on neuronal cells in culture. Br J Pharmacol. 1989 Feb;96(2):480–494. doi: 10.1111/j.1476-5381.1989.tb11841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley P. M., Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol Sci. 1990 May;11(5):205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- Hilton S. M., Redfern W. S. A search for brain stem cell groups integrating the defence reaction in the rat. J Physiol. 1986 Sep;378:213–228. doi: 10.1113/jphysiol.1986.sp016215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C. R., Miljkovic Z., MacDonald J. F. Ketamine and phencyclidine cause a voltage-dependent block of responses to L-aspartic acid. Neurosci Lett. 1985 Oct 24;61(1-2):135–139. doi: 10.1016/0304-3940(85)90414-8. [DOI] [PubMed] [Google Scholar]
- Honoré T. Excitatory amino acid receptor subtypes and specific antagonists. Med Res Rev. 1989 Jan-Mar;9(1):1–23. doi: 10.1002/med.2610090102. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kao M. C., Lee H. K., Chai C. Y., Wang Y. NMDA antagonists attenuate hypertension induced by carotid clamping in the rostral ventrolateral medulla of rats. Brain Res. 1991 May 17;549(1):83–89. doi: 10.1016/0006-8993(91)90602-r. [DOI] [PubMed] [Google Scholar]
- Kihara M., Misu Y., Kubo T. Release by electrical stimulation of endogenous glutamate, gamma-aminobutyric acid, and other amino acids from slices of the rat medulla oblongata. J Neurochem. 1989 Jan;52(1):261–267. doi: 10.1111/j.1471-4159.1989.tb10926.x. [DOI] [PubMed] [Google Scholar]
- Kubo T., Kihara M. Evidence of N-methyl-D-aspartate receptor-mediated modulation of the aortic baroreceptor reflex in the rat nucleus tractus solitarii. Neurosci Lett. 1988 Apr 22;87(1-2):69–74. doi: 10.1016/0304-3940(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Kubo T., Nagura J., Kihara M., Misu Y. Cardiovascular effects of L-glutamate and gamma-aminobutyric acid injected into the rostral ventrolateral medulla in normotensive and spontaneously hypertensive rats. Arch Int Pharmacodyn Ther. 1986 Jan;279(1):150–161. [PubMed] [Google Scholar]
- Le Galloudec E., Merahi N., Laguzzi R. Cardiovascular changes induced by the local application of glutamate-related drugs in the rat nucleus tractus solitarii. Brain Res. 1989 Dec 4;503(2):322–325. doi: 10.1016/0006-8993(89)91683-1. [DOI] [PubMed] [Google Scholar]
- Lodge D., Johnson K. M. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol Sci. 1990 Feb;11(2):81–86. doi: 10.1016/0165-6147(90)90323-z. [DOI] [PubMed] [Google Scholar]
- Lown B., Verrier R. L., Rabinowitz S. H. Neural and psychologic mechanisms and the problem of sudden cardiac death. Am J Cardiol. 1977 May 26;39(6):890–902. doi: 10.1016/s0002-9149(77)80044-1. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Nowak L. M. Mechanisms of blockade of excitatory amino acid receptor channels. Trends Pharmacol Sci. 1990 Apr;11(4):167–172. doi: 10.1016/0165-6147(90)90070-O. [DOI] [PubMed] [Google Scholar]
- Martin D., Lodge D. Ketamine acts as a non-competitive N-methyl-D-aspartate antagonist on frog spinal cord in vitro. Neuropharmacology. 1985 Oct;24(10):999–1003. doi: 10.1016/0028-3908(85)90128-5. [DOI] [PubMed] [Google Scholar]
- Mills E. H., Minson J. B., Pilowsky P. M., Chalmers J. P. N-methyl-D-aspartate receptors in the spinal cord mediate pressor responses to stimulation of the rostral ventrolateral medulla in the rat. Clin Exp Pharmacol Physiol. 1988 Feb;15(2):147–155. doi: 10.1111/j.1440-1681.1988.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Mills E., Minson J., Drolet G., Chalmers J. Effect of intrathecal amino acid receptor antagonists on basal blood pressure and pressor responses to brainstem stimulation in normotensive and hypertensive rats. J Cardiovasc Pharmacol. 1990 Jun;15(6):877–883. doi: 10.1097/00005344-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- SAWYER C. H., EVERETT J. W., GREEN J. D. The rabbit diencephalon in stereotaxic coordinates. J Comp Neurol. 1954 Dec;101(3):801–824. doi: 10.1002/cne.901010307. [DOI] [PubMed] [Google Scholar]
- Sundaram K., Murugaian J., Sapru H. Cardiac responses to the microinjections of excitatory amino acids into the intermediolateral cell column of the rat spinal cord. Brain Res. 1989 Mar 13;482(1):12–22. doi: 10.1016/0006-8993(89)90537-4. [DOI] [PubMed] [Google Scholar]
- Tibirica E., Monassier L., Feldman J., Brandt C., Verdun A., Bousquet P. Baclofen prevents the increase of myocardial oxygen demand indexes evoked by the hypothalamic stimulation in rabbits. Naunyn Schmiedebergs Arch Pharmacol. 1993 Aug;348(2):164–171. doi: 10.1007/BF00164794. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]
- Young A. B., Fagg G. E. Excitatory amino acid receptors in the brain: membrane binding and receptor autoradiographic approaches. Trends Pharmacol Sci. 1990 Mar;11(3):126–133. doi: 10.1016/0165-6147(90)90199-i. [DOI] [PubMed] [Google Scholar]


