Abstract
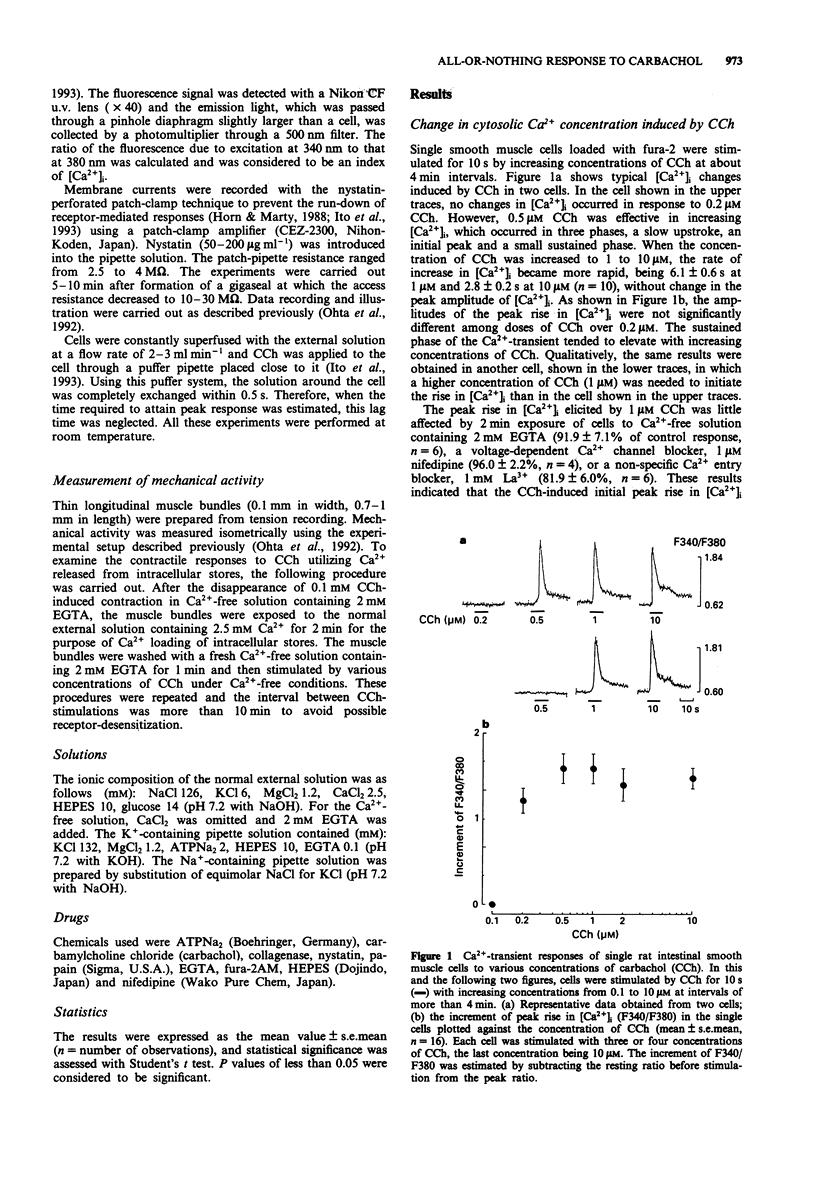
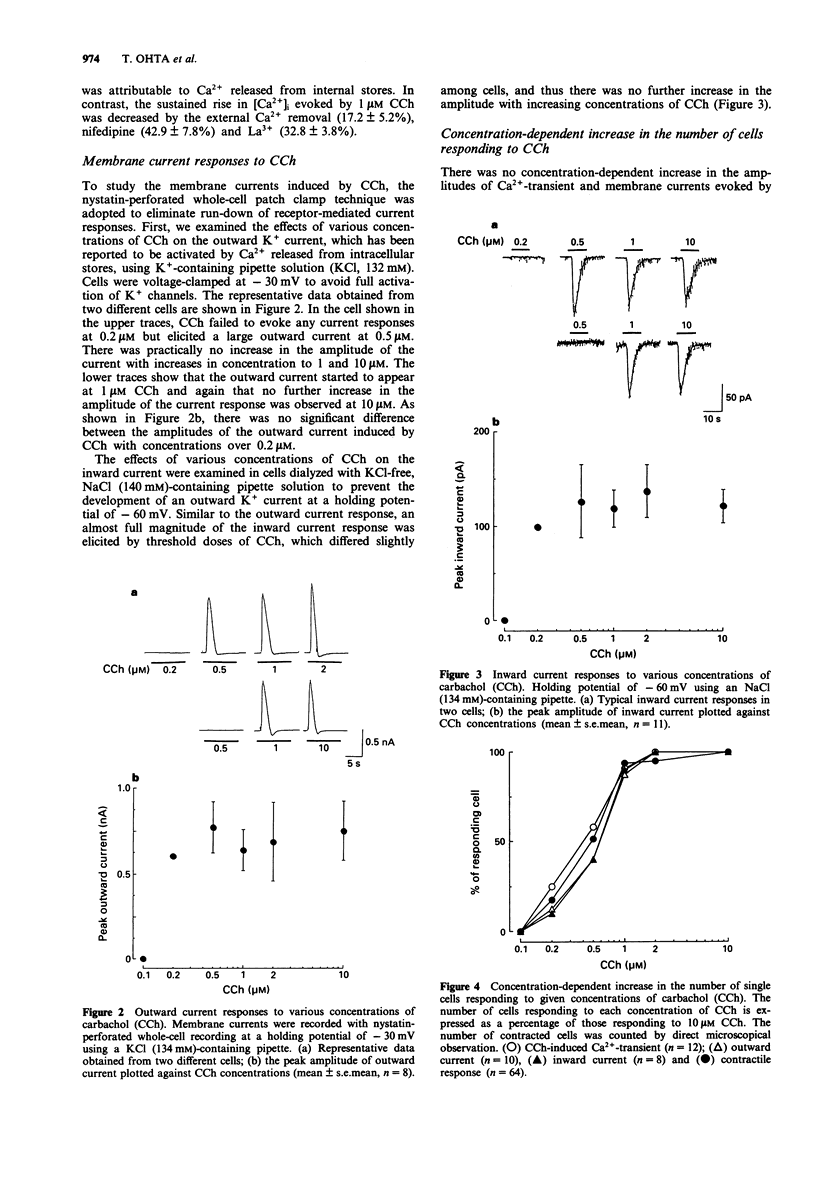
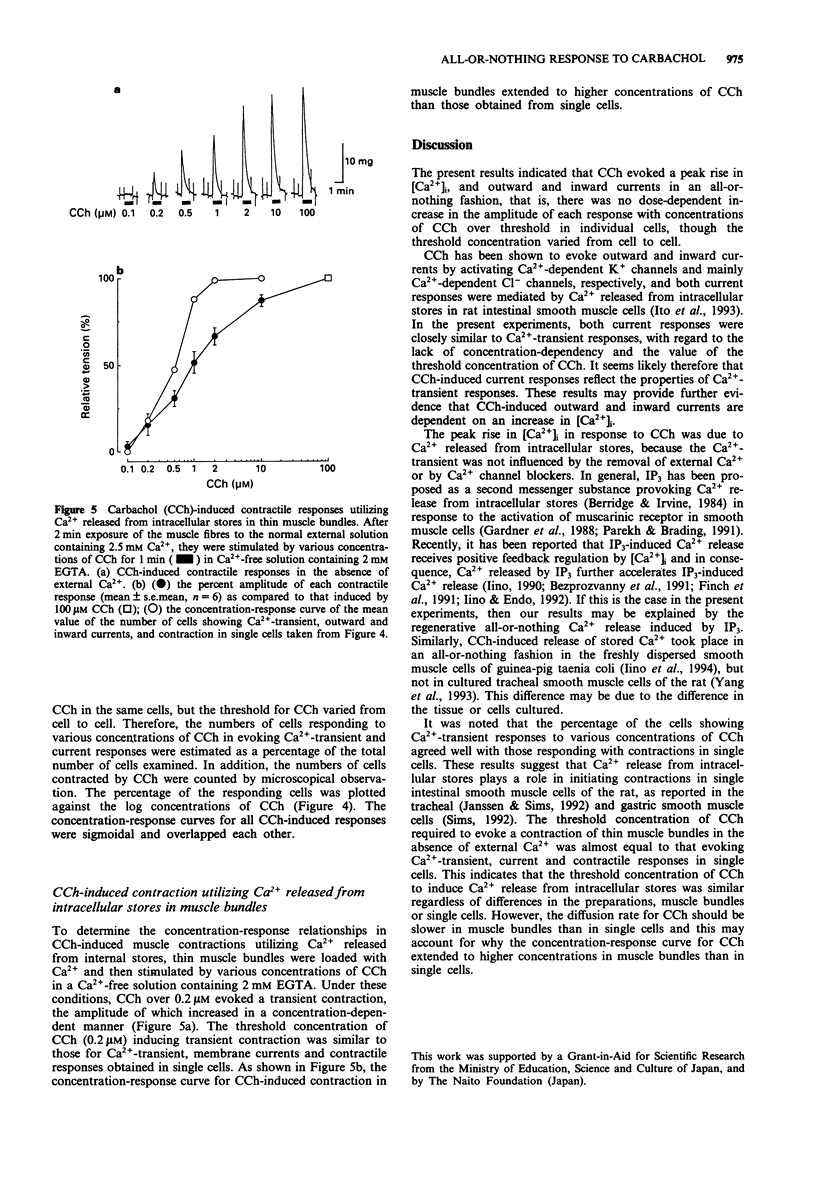
1. Concentration-response relationships for carbachol (CCh)-induced increases in the cytosolic calcium concentration ([Ca2+]i) and membrane currents were studied by use of fura-2 microfluorimetry and nystatin-perforated whole-cell recording in single smooth muscle cells isolated from rat intestine. 2. CCh produced an initial peak rise in [Ca2+]i followed by a small sustained rise. In individual cells, the peak rise in [Ca2+]i did not increase in amplitude even with increasing concentrations of CCh, though the threshold concentration varied in different cells. The initial peak rise in [Ca2+]i, but not the sustained rise, was due to the release of stored Ca2+, because it was unchanged after removal of external Ca2+ and the addition of nifedipine (1 microM) or La3+ (1 mM). 3. CCh elicited an outward and inward current in a cell dialyzed with a pipette solution containing KCl at a holding potential of -30 mV and with one containing NaCl at -60 mV, respectively. In individual cells, the amplitude of each current was similar in cells stimulated at over the threshold concentration of CCh, but the threshold was different among cells. 4. The percentage of cells showing Ca(2+)-transient responses to CCh at given concentrations was similar to those showing current responses and contractile responses. 5. In thin muscle bundles, a concentration-dependent contraction was evoked by CCh in the absence of external Ca2+. Its threshold was similar to those of Ca(2+)-transient and current responses in single cells. 6. These results suggest that CCh-induced release of stored Ca2+ takes place in an all-or-nothing fashion in individual cells of the rat intestinal smooth muscle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Potassium, chloride and non-selective cation conductances opened by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Apr;423:551–568. doi: 10.1113/jphysiol.1990.sp018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lim S. P. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989 Feb;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988 Oct;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle S., Welsh M. J. Inositol trisphosphate is required for the propagation of calcium waves in Xenopus oocytes. J Biol Chem. 1992 Apr 25;267(12):7963–7966. [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Gardner A. L., Choo L. K., Mitchelson F. Comparison of the effects of some muscarinic agonists on smooth muscle function and phosphatidylinositol turnover in the guinea-pig taenia caeci. Br J Pharmacol. 1988 May;94(1):199–211. doi: 10.1111/j.1476-5381.1988.tb11516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Iino M., Yamazawa T., Miyashita Y., Endo M., Kasai H. Critical intracellular Ca2+ concentration for all-or-none Ca2+ spiking in single smooth muscle cells. EMBO J. 1993 Dec 15;12(13):5287–5291. doi: 10.1002/j.1460-2075.1993.tb06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Ohta T., Nakazato Y. Inward current activated by carbachol in rat intestinal smooth muscle cells. J Physiol. 1993 Oct;470:395–409. doi: 10.1113/jphysiol.1993.sp019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990 Aug;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Yuzaki M., Nakada K., Shirakawa H., Nakanishi S., Nakade S., Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992 Jul 10;257(5067):251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Ohta T., Ito S., Nakazato Y. Chloride currents activated by caffeine in rat intestinal smooth muscle cells. J Physiol. 1993 Jun;465:149–162. doi: 10.1113/jphysiol.1993.sp019670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Ito S., Noto T., Tachibana R., Nakazato Y., Ohga A. The inhibitory action of cyclic AMP on responses to carbachol dependent on calcium stores in rat gastric smooth muscle. J Physiol. 1992;453:367–384. doi: 10.1113/jphysiol.1992.sp019233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Mironneau C., Mironneau J. Noradrenaline activates a calcium-activated chloride conductance and increases the voltage-dependent calcium current in cultured single cells of rat portal vein. Br J Pharmacol. 1989 May;97(1):139–146. doi: 10.1111/j.1476-5381.1989.tb11934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B., Brading A. F. The sources of calcium for carbachol-induced contraction in the circular smooth muscle of guinea-pig stomach. Br J Pharmacol. 1991 Oct;104(2):412–418. doi: 10.1111/j.1476-5381.1991.tb12444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims S. M. Cholinergic activation of a non-selective cation current in canine gastric smooth muscle is associated with contraction. J Physiol. 1992 Apr;449:377–398. doi: 10.1113/jphysiol.1992.sp019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. M., Yo Y. L., Wang Y. Y. Intracellular calcium in canine cultured tracheal smooth muscle cells is regulated by M3 muscarinic receptors. Br J Pharmacol. 1993 Nov;110(3):983–988. doi: 10.1111/j.1476-5381.1993.tb13910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


