Abstract
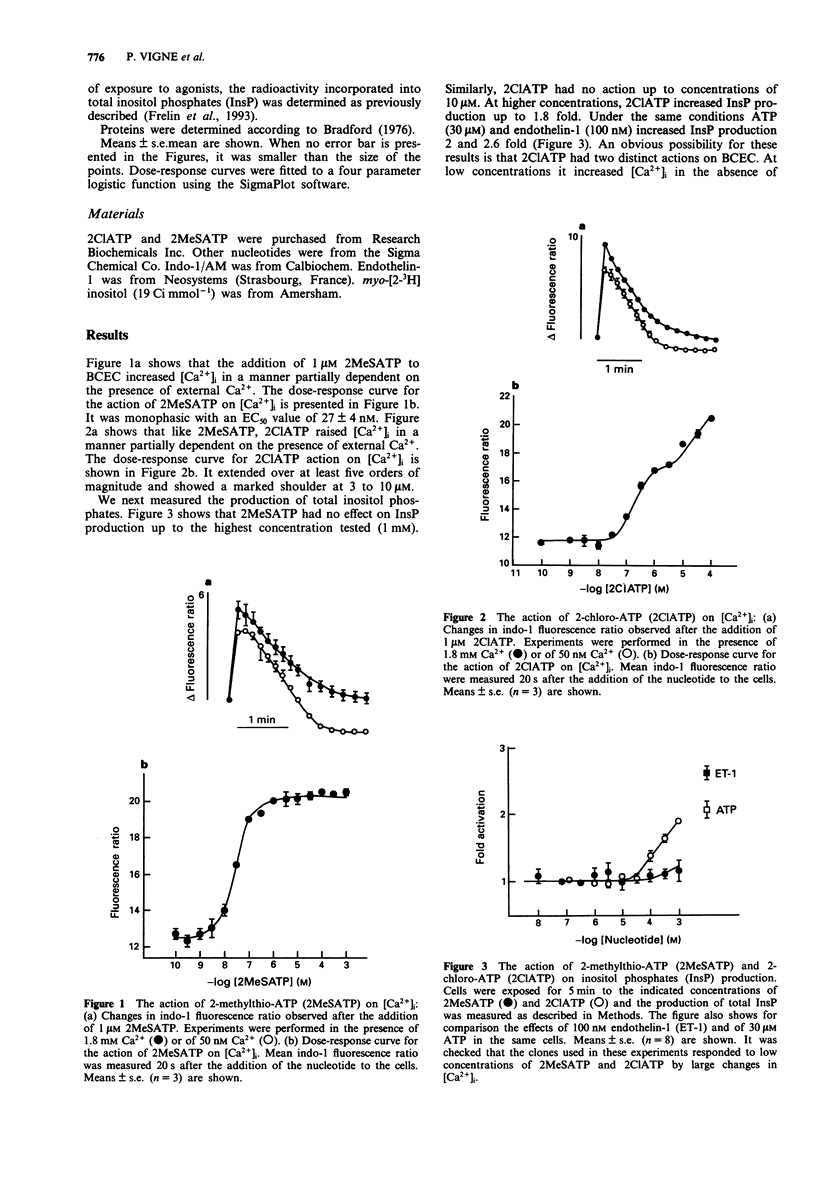
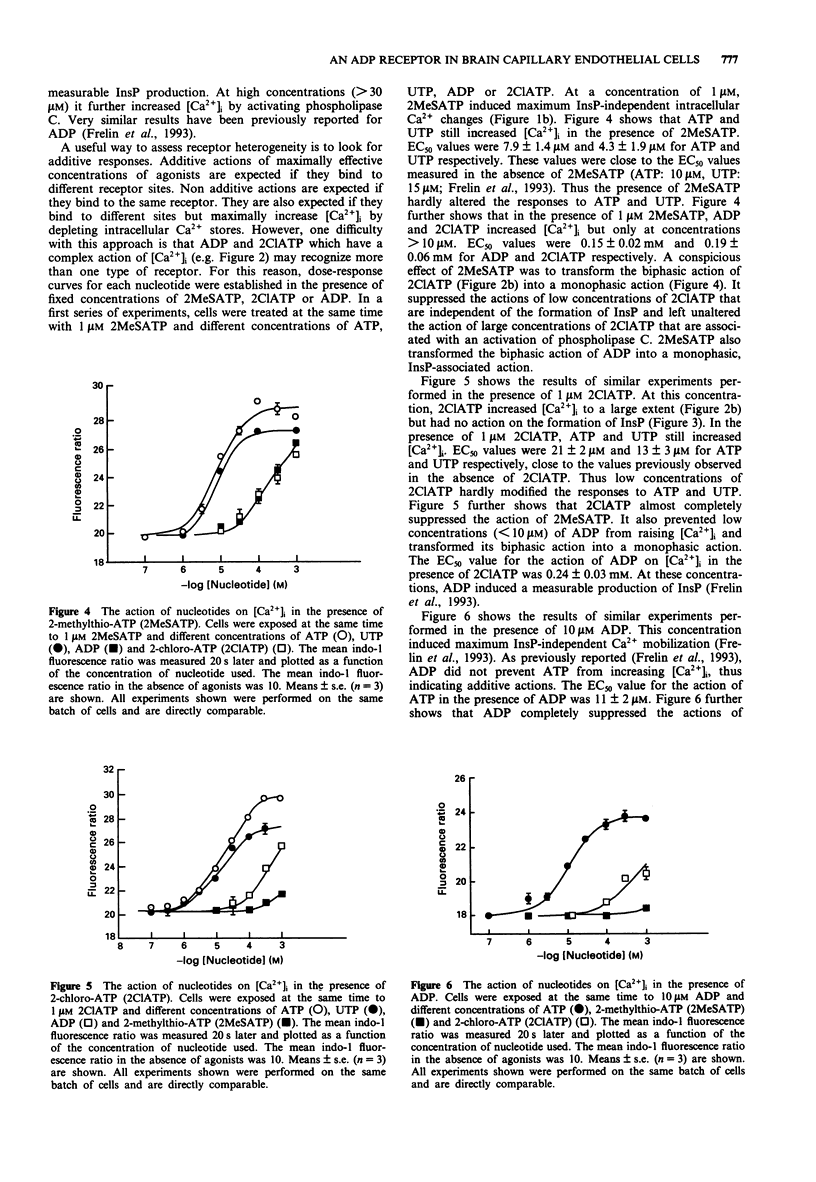
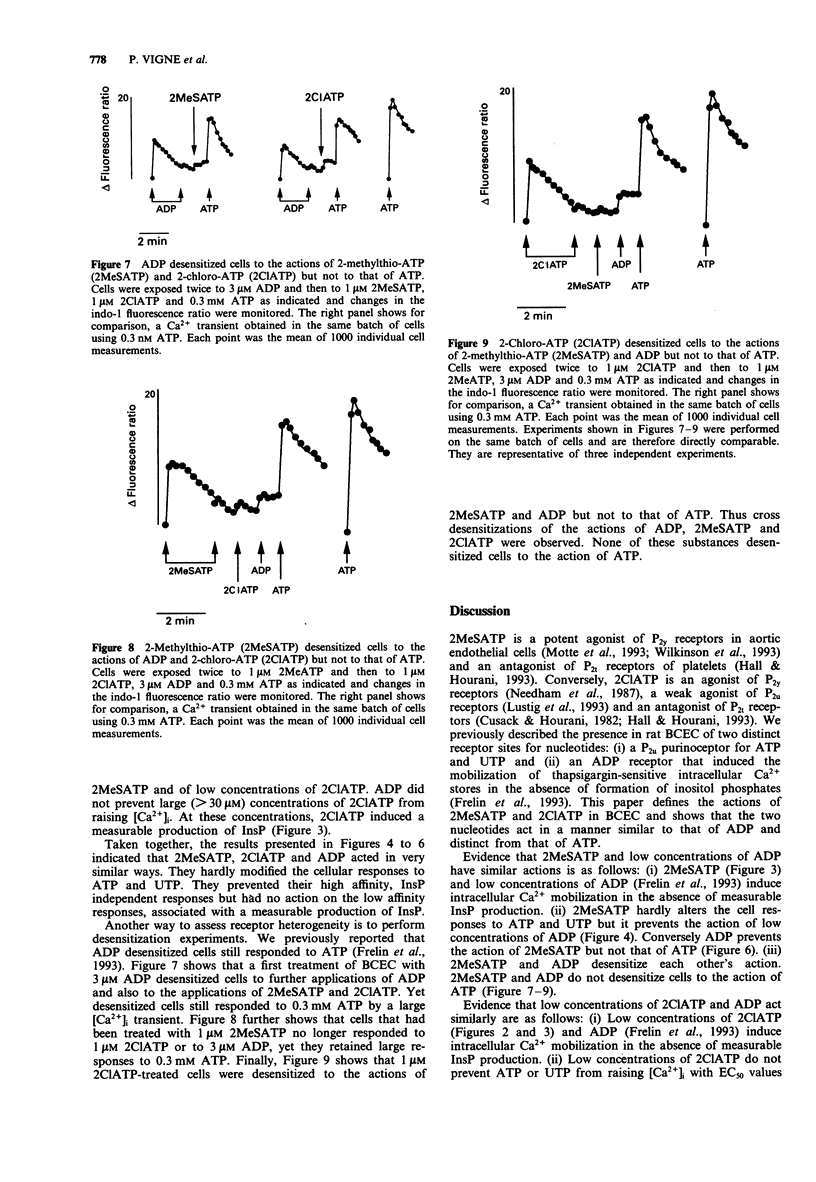
1. Brain capillary endothelial cells responded to 2-methylthio-ATP (2MeSATP) by large increases in [Ca2+]i (EC50 = 27 nM) that were partially dependent on the presence of extracellular Ca2+ and that were not associated with a measurable production of inositol phosphates. 2. 2-chloro-ATP (2ClATP) raised [Ca2+]i in a biphasic manner. At low concentrations, intracellular Ca2+ mobilization was not associated with a measurable production of inositol phosphates. At concentrations > 30 microM, 2ClATP activated phospholipase C. 3. The actions of 2ClATP, 2MeSATP and ADP on [Ca2+]i were additive to those of ATP and UTP. Non-additive actions of 2MeSATP and of low concentrations of ADP or of 2ClATP were observed. 4. Cross desensitizations of the actions of ADP, 2MeSATP and 2ClATP were observed. None of them desensitized cells to the action of ATP. 5. It is concluded that 2MeSATP and low concentrations of 2ClATP and ADP induce intracellular Ca2+ mobilization by acting via an atypical P2y purinoceptor that is not coupled to phospholipase C. At high concentrations, 2ClATP also activates phospholipase C and further increases [Ca2+]i probably by acting on P2u purinoceptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown H. A., Lazarowski E. R., Boucher R. C., Harden T. K. Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5'-nucleotide receptor in human airway epithelial cells. Mol Pharmacol. 1991 Nov;40(5):648–655. [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M. Specific but noncompetitive inhibition by 2-alkylthio analogues of adenosine 5'-monophosphate and adenosine 5'-triphosphate of human platelet aggregation induced by adenosine 5'-diphosphate. Br J Pharmacol. 1982 Feb;75(2):397–400. doi: 10.1111/j.1476-5381.1982.tb08800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C. J., Woods N. M., Cuthbertson K. S., Cobbold P. H. Evidence for two Ca2(+)-mobilizing purinoceptors on rat hepatocytes. Biochem J. 1990 Jul 15;269(2):499–502. doi: 10.1042/bj2690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J., Cole P., Davidson J. S. Extracellular nucleotides stimulate receptor-mediated calcium mobilization and inositol phosphate production in human fibroblasts. Biochem J. 1989 Oct 15;263(2):371–376. doi: 10.1042/bj2630371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Breittmayer J. P., Vigne P. ADP induces inositol phosphate-independent intracellular Ca2+ mobilization in brain capillary endothelial cells. J Biol Chem. 1993 Apr 25;268(12):8787–8792. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. A., Hourani S. M. Effects of analogues of adenine nucleotides on increases in intracellular calcium mediated by P2T-purinoceptors on human blood platelets. Br J Pharmacol. 1993 Mar;108(3):728–733. doi: 10.1111/j.1476-5381.1993.tb12869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Characterization of the biological effects of 2-methylthio-ATP on rat hepatocytes: clear-cut differences with ATP. Br J Pharmacol. 1991 Oct;104(2):301–304. doi: 10.1111/j.1476-5381.1991.tb12426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandekerckhove A., De Wulf H. Characterization of the effects of adenosine 5'-[beta-thio]-diphosphate in rat liver. Br J Pharmacol. 1993 Mar;108(3):663–668. doi: 10.1111/j.1476-5381.1993.tb12858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandekerckhove A., De Wulf H. Extracellular ATP and UTP exert similar effects on rat isolated hepatocytes. Br J Pharmacol. 1992 Feb;105(2):475–479. doi: 10.1111/j.1476-5381.1992.tb14278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte S., Pirotton S., Boeynaems J. M. Heterogeneity of ATP receptors in aortic endothelial cells. Involvement of P2y and P2u receptors in inositol phosphate response. Circ Res. 1993 Mar;72(3):504–510. doi: 10.1161/01.res.72.3.504. [DOI] [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Comparison of extracellular ATP and UTP signalling in rat renal mesangial cells. No indications for the involvement of separate purino- and pyrimidino-ceptors. Biochem J. 1990 Dec 1;272(2):469–472. doi: 10.1042/bj2720469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha S., de Souza L. R., Reed J. K. Intracellular signalling by nucleotide receptors in PC12 pheochromocytoma cells. J Cell Physiol. 1993 Mar;154(3):623–630. doi: 10.1002/jcp.1041540322. [DOI] [PubMed] [Google Scholar]
- Vigne P., Champigny G., Marsault R., Barbry P., Frelin C., Lazdunski M. A new type of amiloride-sensitive cationic channel in endothelial cells of brain microvessels. J Biol Chem. 1989 May 5;264(13):7663–7668. [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. F., Purkiss J. R., Boarder M. R. The regulation of aortic endothelial cells by purines and pyrimidines involves co-existing P2y-purinoceptors and nucleotide receptors linked to phospholipase C. Br J Pharmacol. 1993 Mar;108(3):689–693. doi: 10.1111/j.1476-5381.1993.tb12862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


