Abstract
Pulmonary surfactant, secreted via exocytosis of lamellar bodies (LB) by alveolar type II (AT II) cells, maintains low alveolar surface tension and is therefore essential for normal lung function. Here we describe real-time monitoring of exocytotic activity in these cells by visualizing and quantifying LB fusion with the plasma membrane (PM). Two approaches were used. First, fluorescence of LysoTracker Green DND-26 (LTG) in LB disappeared when the dye was released after exocytosis. Second, phospholipid staining by FM 1–43 resulted in bright fluorescence when this dye entered the LB through the fusion pore. Both processes were restricted to and colocalized with LB and occurred simultaneously. In AT II cells, FM 1–43 offered the unique advantage to independently define the moment and cellular location of single exocytotic events as well as the amount of material released, and to monitor its extracellular fate. Furthermore, both dyes could be used in combination with fura-2. The results indicate considerable diversity in the dynamics of LB exocytosis. In the majority of cells stimulated with ATP and isoproterenol, the first fusion of LB coincided with the rise of [Ca2+]i, but subsequent response of other LB in the same cell considerably outlasted this signal. In other cells, however, the onset of exocytosis was delayed by several minutes. After LB fusion, release of surfactant from LB into an aqueous solution was slow. In summary, stimulated exocytosis in AT II cells occurs at a much slower rate than in most other secretory cells but is still a more dynamic process than predicted from conventional measurements of surfactant released into cell supernatants.
A common feature of exocytotic processes is the fusion of discrete intracellular organelles with the PM by highly conserved mechanisms (1, 2). In the lung the major product secreted by exocytosis is surfactant, a complex, phospholipid-rich, lipoprotein-like material lining the respiratory surface. By lowering surface tension at the air–liquid interface, surfactant is an important determinant of lung compliance and prevents alveolar collapse. It may have the additional functions of preventing lung edema and assisting inflammatory responses (3, 4). Deficiency or dysfunction of surfactant results in severe lung disease, the best example of which is respiratory distress syndrome of newborns. Failure of surfactant secretion or metabolism of different etiologies may also be a major cause of adult respiratory distress syndrome (5, 6).
Almost all constituents of surfactant are stored in and released from intracellular vesicles termed lamellar bodies (LB) by AT II cells. Several lines of evidence indicate the following signaling pathways to play an important role in the regulation of secretion: formation of cAMP, activation of PKC, and Ca2+-dependent processes (reviewed in refs. 7–10). These pathways may be activated by neurotransmitters, local mediators, hormones, but also by physical or chemical factors, the best understood being mechanical stretch (11). In the isolated AT II cell the mechanisms of β-adrenergic and purinergic stimulation have been most extensively studied. Pharmacological evidence suggests that β2-adrenergic stimulation activates the formation of cAMP (12) but also elicits a transient increase of [Ca2+]i (13). The actions of ATP are more complex and involve both P1 receptor-dependent cAMP formation (14) as well as P2 receptor-dependent phosphoinositide hydrolysis and subsequent Ca2+ mobilization (15–17). The actions of Ca2+ and PKC and those of cAMP are additive, supporting the notion that surfactant secretion is independently regulated at various sites (18, 19).
A key process in surfactant secretion is exocytosis of LB, and there have been several ultrastructural observations of lamellar material passing from AT II cells into alveoli (reviewed in ref. 7). In this report we define exocytosis as the fusion of LB with the PM but not as the subsequent release of stored material into the extracellular fluid. It is obvious that regulation of surfactant secretion may take place at different intracellular steps, including movement of LB to the cell surface, and their adhesion to and, finally, their fusion with the PM. Consistently, actin or microtubule disrupting agents have been shown to interfere with surfactant secretion (20–22). Furthermore, synexin (23, 24) and annexin II (25) promote fusion of isolated LB with membranes, and annexin II is effective in reconstituting phosphatidylcholine secretion in permeabilized AT II cells (26).
Despite the obvious importance of LB exocytosis in the control of surfactant secretion, this has not yet been directly observed but has been measured primarily by the release of radiolabeled phospholipid or phosphatidylcholine. In all these studies the implicit conclusion would be that stimulated secretion is the result of stimulated exocytosis. These methods based on bulk measurements of secreted material are, however, not without some major drawbacks. (i) They have a poor time resolution, and determination of the onset of secretion is vague. (ii) They yield average responses of a whole cell population, ignoring the amount of nonresponding cells, and allow only limited conclusions about individual cells. (iii) Because diffusion of LB content into an aqueous environment could be a limiting factor, reliable conclusions about the time course of exocytosis cannot be drawn. For these reasons, direct observation of exocytosis in single AT II cells would be most promising to further elucidate the mechanisms involved therein.
In this study we demonstrate continuous measurement of exocytosis at the level of single LB in living AT II cells. Our data show that the onset of exocytosis on agonist stimulation essentially coincides with intracellular Ca2+ release in the majority of cells. Within a single cell, however, exocytosis of most other LB is considerably delayed, outlasting the Ca2+ signal but exhibiting its highest activity during the first few minutes of stimulation. In contrast, diffusion of surfactant into the bathing solution is an even slower and eventually incomplete process.
METHODS
Cell Preparation.
AT II cells were isolated from anesthetized (thiopental) male Sprague–Dawley rats (≈200 g), according to the procedure of Dobbs et al. (27). Briefly, lungs were cleared of blood by perfusion and removed from the thorax. After lavage, the lungs were instilled with elastase solution (30 units/ml), incubated at 37°C, and minced with scissors in the presence of DNase. After stopping the elastase reaction by addition of fetal calf serum (FCS), the cell suspension was sequentially filtered through cotton gaze and three nylon meshes (150-, 20-, and 10-μm mesh size) and centrifuged at 900 rpm for 8 min. The cell pellet was resuspended in DMEM and panned on an IgG-coated plastic dish at 37°C to remove macrophages. The unattached cells were centrifuged, suspended in DMEM supplemented with 10% FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 24 mmol/l NaHCO3, seeded on glass coverslips at low density (40 cells per mm2), and cultured in 95% humidified air and 5% CO2 at 37°C. Cells were used 1–2 days thereafter.
Fluorescence Staining.
For dye loading, the cells were preincubated at 37°C in DMEM with LTG (50 nmol/liter; 30 min) or fura-2 AM (1.2 μmol/liter, 15 min; both dyes were purchased from Molecular Probes). Coverslips with stained cells were mounted into a perfusion chamber placed on the stage of an inverted microscope and rinsed at 25°C with bath solution (140 mmol/liter NaCl, 5 mmol/liter KCl, 1 mmol/liter MgCl2, 2 mmol/liter CaCl2, 5 mmol/liter glucose, 10 mmol/liter Hepes, pH 7.4). Exocytosed surfactant was stained in the continuous presence of 4 μmol/liter FM 1–43 (Molecular Probes) in the nonperfused bath solution.
Fluorescence Measurements.
The microscope was equipped for epifluorescence and photometry as described (28). Briefly, light from a xenon arc lamp was diffracted by a Jobin Yvon holographic mirror (Longjumeau, France), providing consecutive light flashes (20 ms) with 340, 380 nm (fura-2) and—unless stated otherwise—490 nm wavelength (LTG and FM 1–43), respectively, followed by 1 s of darkness. The excitation light was directed through a quartz glass fiber and a gray filter (3% nominal transmission) into the microscope. Excitation light was deflected by a 520-nm dichroic mirror into a Plan-Neofluar 100× oil objective. For simultaneous fura-2 and FM 1–43 measurements, a Fluar 40× oil objective was used. Mutual interferences between FM 1–43 fluorescence and the fura-2 fluorescence ratio were negligible. The emitted light was directed through a 520-nm cut-off filter to a photomultiplier tube. Dual-emission photometry was performed by an emission-beam splitter (TILL Photonics, Planegg, Germany) and two photomultiplier tubes (H5784 Hamamatsu, Herrsching, Germany), equipped with a 500-nm (21 nm half-width) interference and a 590-nm cut-off filter (LOT-Oriel, Langenberg, Germany), respectively. A pin hole in the image plane of the phototube reduced the region from which fluorescence was collected. Thus, measured fluorescence originated from single cells. Images in Fig. 1 were taken with an integrating cooled charge-coupled device connected to an inverted microscope (135 TV, Zeiss) video camera. Photographs in Fig. 3 were made on Kodak Ektachrome P1600x processed for 3200 ASA. For two-dimensional fluorescence analysis in Fig. 6, a video imaging system, connected to a 135 TV inverted microscope (Zeiss) equipped essentially as above, was used. Fluorescence images were acquired with a Neofluar 100× oil objective (Zeiss) by a Peltier cooled slow-scan camera (SIS 90, Thetha Systems). Image analysis was performed with the software fucal (TILL Photonics) and datgraf (Cyclobios, Innsbruck, Austria). Data are reported as arithmetic means ± SEM.
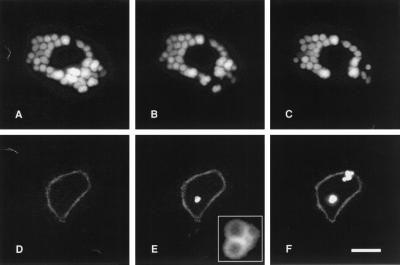
Figure 1.
Consecutive video images of two single AT II cells stained with LTG (A–C) or FM 1–43 (D–F). (A) Nonstimulated cell preincubated with LTG. Bright fluorescence resulted from accumulation of LTG in LB. Same cell 10 min (B) and 30 min (C) after stimulation with ATP and isoproterenol: LTG-fluorescence completely disappeared in a restricted area of the cell only. Images were corrected for small intensity loss of LTG fluorescence because of photobleaching. (D) Nonstimulated cell in the presence of FM 1–43. Only the PM was weakly stained. Same cell 10 min (E) and 30 min (F) after stimulation. During these periods, bright spots appeared as clusters. Fluorescence resulted from FM 1–43 incorporating into lipid LB content. The weak PM staining in D–F was increased by contrast enhancement. Inset in E is a magnification of the same group of exocytosed LB (note the dark core and bright periphery of each LB). (Bar = 10 μm.)
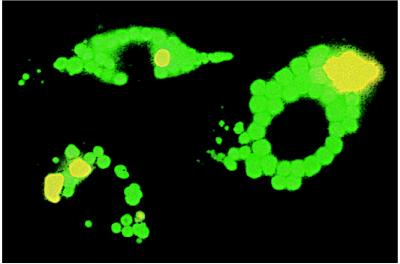
Figure 3.
Photographs of three AT II cells colabeled with LTG (green) and FM 1–43 (dark yellow), 30 min after stimulation with ATP and isoproterenol. LTG accumulated in pre-exocytotic LB, whereas FM 1–43 specifically stained LB content after fusion with the PM. Image size: 40 × 60 μm.
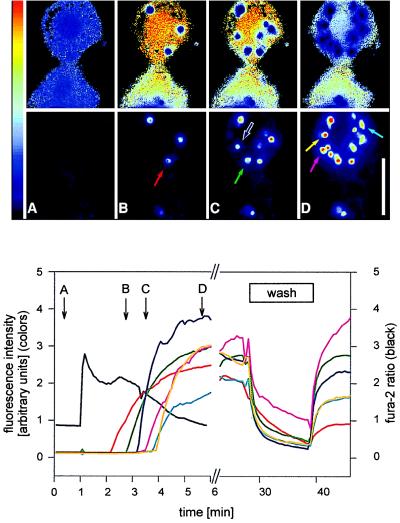
Figure 6.
Simultaneous fluorescence measurements of fura-2 and localized FM 1–43 in single AT II cells. (Upper) Pseudocolor video images of two cells taken at times A, B, C, and D in the bottom graph. Upper sequence: fura-2 ratio. LB appear as dark areas (A). The pronounced appearance of dark spots in B–D results from FM 1–43 interference with the fura-2 ratio calculation. Lower sequence: 460 nm excitation. The images show that fura-2 did not interfere with FM 1–43 fluorescence. The dark picture in A indicates lack of exocytosed material before stimulation. Colored arrows mark the appearance of stained vesicles because of exocytosis of LB. The time course of FM 1–43 fluorescence intensity of each marked LB is shown in the graph (Lower). (Lower) Kinetic analysis derived from the Upper images. 1 min = addition of ATP and isoproterenol to the bath. The transient increase of the fura-2 ratio (black line) reflects release of Ca2+ from intracellular stores. Fluorescence intensities of FM 1–43 (colored lines) were measured from the areas of respective LB, indicated by colored arrows in the top images. Removal of FM 1–43 by superfusion with control solution (“wash”) diminished localized FM 1–43 fluorescence. This decrease was primarily due to diffusion of FM 1–43 out of the vesicles but not to washout of lipid material, because readdition of FM 1–43 to the nonperfused bath (at the end of “wash”) resulted in an immediate restaining. This indicates that even under high bath perfusion rates, exocytosed LB material remains firmly attached to the cell. (Bar = 10 μm.)
RESULTS
Visualizing LB in Vital AT II Cells.
Aiming at optical tracking of LB, we found the fluorescent dye LTG to selectively stain these organelles in primary culture AT II cells. As an uncharged compound, LTG is freely permeant to cell membranes and accumulates in acidic compartments by a process involving diffusion and trapping by protonation (28). LB, like many other secretory granules, are known to have a pH of 6.1 or below maintained by an energy-dependent process (29). Accumulation of LTG in LB yielded bright green fluorescence (Figs. 1 A–C and 3), whereas emission intensity (Fig. 4) and spectral properties (data not shown) of the dye itself were essentially pH-independent. Although LTG also accumulates in other acidic compartments (28), emitted LTG-fluorescence in AT II cells originated almost exclusively from LB. Because LB could be viewed by transmission microscopy owing to their characteristic shape and proportions, we found that LTG fluorescence strictly colocalized with LB and that background fluorescence was negligible (Figs. 1 A–C and 3). The effective labeling of and retention within LB by LTG enabled long-term tracing of these organelles in living AT II cells.
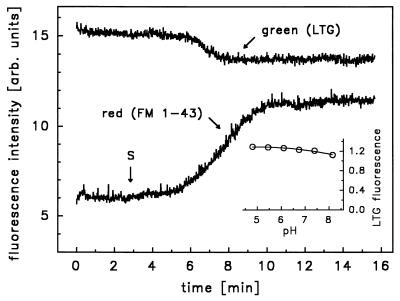
Figure 4.
Simultaneous measurement of green (500 ± 21 nm) and red (>590 nm) fluorescence at 470 nm excitation in a single cell preloaded with LTG and in the presence of FM 1–43. Green fluorescence primarily originated from LTG [emission maximum (em. max.), 511 nm] and red fluorescence originated from FM 1–43, because emission intensity of LTG is negligible at λ > 590 nm. S, stimulation with ATP and isoproterenol. (Inset) pH dependence of LTG fluorescence intensity in 100 mmol/liter phosphate-buffered solution. Exc. = 490 nm; em. = 530 nm. SEM smaller than symbols (n = 5).
Loss of LTG-Stained LB by Stimulation.
In unstimulated AT II cells, the number of LTG-labeled LB per cell remained essentially constant over an observed period of 90 min (Fig. 2). In contrast, a scattered disappearance of LTG fluorescence occurred upon a combined application of ATP and isoproterenol (both 10 μmol/liter) in the perfusate (Figs. 1 A–C and 2). We used this method to stimulate AT II cells in all experiments because the additive actions of Ca2+, PKC, and cAMP on surfactant secretion yield maximum responses. As discussed below, the disappearance of LTG fluorescence cannot be explained by a putative breakdown of the H+ gradient but only by diffusion of the dye into the perfusate after exocytosis of LB.
Figure 2.
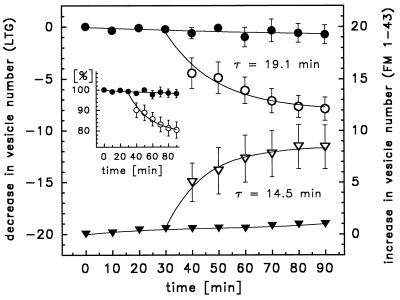
Decrease in the number of LTG-stained vesicles (circles, left ordinate) versus increase in the number of FM 1–43-stained vesicles (triangles, right ordinate) in nonstimulated (solid symbols) and stimulated (open symbols) cells. ATP and isoproterenol were added to the nonperfused bath immediately after 30 min. Both staining methods yielded essentially the same number and time course of exocytosed LB. Data were fitted by the equation y(t) = A + B × exp(−t/τ). (Inset) Decrease of LTG-stained vesicles expressed as % of total number of cellular LB. Each value represents the mean of 9 independent experiments, each comprising up to 15 single cell measurements.
Visualizing Post-Exocytotic LB.
Because current knowledge of surfactant secretion is limited yet suggests a rather slow process, we aimed at visualizing this at a stage immediately upon fusion of LB with the PM. We found that FM 1–43, a fluorescent dye binding to but not penetrating PM owing to its amphiphilic properties (30), efficiently stained isolated bovine surfactant (unpublished observation). Furthermore, FM 1–43 is nonfluorescent in aqueous solutions but emits fluorescence upon intercalating with lipids. When added to the bath solution, most AT II cells exhibited a weak labeling of the PM because of the insertion of FM 1–43 into the outer leaflet (Fig. 1 D–F). In the majority of viable cells, FM 1–43 fluorescence remained restricted to the cell surface up to 90 min (Fig. 2); longer incubations occasionally resulted in dye leakage into the cell. Stimulation, however, resulted in the appearance of stained vesicles, preferentially as clusters in the cell periphery, with a brightness far exceeding the faint signal of the PM (Figs. 1 D–F, 3, and 6). We hypothesized that this fluorescence originated from FM 1–43 entering the LB through the exocytotic fusion pore, thus staining LB contents. Occasionally, floating tubule-like, stained material protruding from exocytosed LB into the bath could be seen, resembling “tubular myelin” (31). The intensive brightness of stained vesicles is consistent with the known high lipid composition and surface of surfactant in LB. To test this hypothesis, we colabeled AT II cells with LTG and FM 1–43. Fig. 3 is a photograph of such cells after stimulation. Green (LTG) and dark yellow (FM 1–43) spots were identical in size and appearance, but neither overlapped nor exhibited intermediate shades of color. In addition, we independently measured fluorescence at 500 nm and >590 nm by using dual emission photometry. The gain of FM 1–43 fluorescence (>590 nm) occurred almost concomitantly with the loss of LTG fluorescence (500 nm), as shown by a representative single cell measurement in Fig. 4. These observations essentially prove that FM 1–43 specifically stained LB content after fusion with the PM. Thus, treatment with LTG and FM 1–43 allows to discriminate between LB before (pre-exocytotic) and after membrane fusion (post-exocytotic), respectively. Consistently, in cells treated with either dye, the gain of FM 1–43-stained vesicles matched the loss of LTG-stained LB at approximately the same rate (Fig. 2). Importantly, even within 60 min of continued presence of agonists, the slope of the exponential fit (FM 1–43 data) declined to 1.6% of the initial rate (at time 30 min in Fig. 2) and was no longer significantly different from the control, indicating termination of stimulated exocytosis.
Continuous Monitoring of [Ca2+]i and Cumulative Exocytosis.
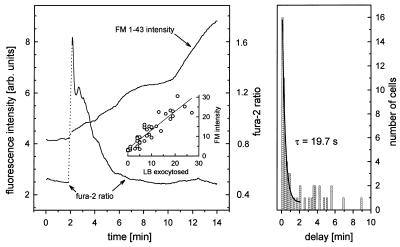
The rapid gain of FM 1–43-stained vesicles during the first 10 min of stimulation, as revealed by counting them from video images captured at 10-min intervals (Figs. 1 and 2), suggested a relatively high initial rate of exocytosis. Because we demonstrated by photometric measurements that total cellular fluorescence intensity of FM 1–43 correlated well with the number of exocytotic events (Fig. 5), we used photometry to continuously monitor cumulative exocytosis in single cells with a better time resolution. Furthermore, simultaneous measurements with fura-2 allowed us to relate the onset of LB exocytosis with changes in [Ca2+]i after treatment with ATP and isoproterenol. Fig. 5 reveals that this onset essentially coincided with the rise of [Ca2+]i or was shortly delayed with a time constant (τ) of 19.7 s when fitted by a single exponential (“fast responders”). Other cells, however, responded with a variable but considerably longer delay (up to several minutes, “slow responders”) (Fig. 5). Nonresponding cells comprised 40–60% of all AT II cells. Once initiated, exocytotic activity continued with τ = 14.5 min (Fig. 2).
Figure 5.
(Left) Simultaneous fluorescence measurements of fura-2 and FM 1–43 in a single AT II cell. The fura-2 ratio (right ordinate) was calculated by dividing emission intensities obtained at 340 and 380 nm exc., respectively, after subtracting background fluorescence (quench by 5 mmol/liter MnCl2) at each wavelength. ATP and isoproterenol were added while the photomultiplier was switched off (dotted lines). Stimulation was followed by a transient and occasionally biphasic increase in [Ca2+]i. Onset of cumulative exocytosis (i.e., onset of FM 1–43 fluorescence increase, left ordinate) coincided with the rise in [Ca2+]i and proceeded stepwise. (Inset) Correlation between number of exocytosed LB and gain of FM 1–43 fluorescence intensity, r = 0.74. (Right) Exocytosis response-time frequency histogram of single AT II cells. Number of cells were binned in 10-s intervals. Delay denotes time between the peak of [Ca2+]i and onset of FM 1–43 fluorescence increase. Values within 2 min could be well fitted by a single exponential, y(t) = A + B × exp(−t/τ).
Resolving Single Exocytotic Events.
Although the increase of FM 1–43 fluorescence, measured over an entire cell, frequently proceeded stepwise (Fig. 5), this approach possibly might not be able to resolve successive exocytotic events of single LB, but rather might be able to resolve those of LB clusters. Video imaging enabled us to simultaneously measure [Ca2+]i and the time course of localized exocytosis by confining the sampling area of FM 1–43 fluorescence to single LB, as shown in Fig. 6. The results confirmed that onset of exocytosis was fast and coincided with the peak [Ca2+]i. This fast response was, however, restricted to a few LB only, whereas other LB in the same cell responded later and in a sequential fashion when [Ca2+]i had declined or already reached basal values. In many instances, LB exocytosed almost synchronously as clusters of two or more. In contrast to the fast beginning of exocytotic activities in AT II cells, surfactant release from exocytosed LB into the surrounding solution was slow and incomplete within 2 hr. Perfusion of the cells (Fig. 6) rapidly diminished FM 1–43 fluorescence, which we explain as a wash-out effect. Readdition of FM 1–43 to the nonperfused bath, however, caused an immediate restaining of most surfactant particles with an intensity nearly as high as before perfusion. From these observations we conclude that in an aqueous solution, surfactant material remains closely attached to the cell surface and cannot easily be removed by stirring or rigorous perfusion of the cells.
DISCUSSION
Methodological Aspects.
The present study describes two complementary methods to visualize and quantify the secretory process in single, living AT II cells by fluorescence imaging techniques.
Pre-exocytotic LB are effectively labeled with LTG, allowing us to track these organelles until they fuse with the PM. Intensive labeling of LB with LTG confirms earlier findings that LB maintain an acidic interior. Although the physiological importance of this low intravesicular pH in LB is not yet clear, it may play a role in membrane trafficking and vesicle fusion (32). It is at the same time a criterion for cell viability, because LB acidification is an energy-dependent process (29). Although we are not aware of morphometric studies quantifying the lysosomal volume of AT II cells, we explain the highly specific LB staining and the weak background fluorescence with the relatively small contribution of lysosomes and other acidic compartments to total cell volume, as described for other cells (33). The disappearance of LTG fluorescence after stimulation could theoretically be explained by a dissipation of the pH gradient across LB and cytosol or subsequent redistribution of the dye within the cell. This possibility can be excluded for the following reasons. (i) We showed that LTG fluorescence intensity is essentially pH-independent (Fig. 4). (ii) LTG fluorescence remained restricted to LB and did not shift into the cytosol, as would be expected in that case. (iii) Complete loss of LTG was localized and restricted to a few LB only. Therefore, photobleaching of LTG can also be ruled out. (iv) Total cellular LTG fluorescence decreased after stimulation, indicating loss of dye. Hence, exocytosis of LTG and subsequent dilution of the dye in the perfusate is the only reasonable explanation for its disappearance in a restricted number of LB.
Post-exocytotic LB are specifically stained as soon as extracellular FM 1–43 gets access to open LB and inserts into lipids. The latter is a prerequisite for FM 1–43 to emit light, because the dye is virtually nonfluorescent in aqueous solutions. Part of this fluorescence is expected to originate from dye insertion into the expanded membrane surface after exocytosis, and the other part is expected to originate from insertion into lipid surfactant material. In fact, the property of FM 1–43 to detect a gain of PM after vesicle fusion was used in investigations about exocytosis and endocytosis in other cell types (30, 34). Considering the weak labeling of the PM in unstimulated cells, however, the appearance of bright spots in stimulated AT II cells cannot be solely explained by the restricted increase of the PM surface. Furthermore, isolated bovine surfactant proved highly fluorescent when added to an FM 1–43-containing solution (unpublished observation). Several other observations also support our notion that FM 1–43 primarily stained surfactant material and, only to a negligible extent, the PM: (i) the appearance of fluorescent, floating, tubule-like structures, originating from exocytosed LB, which resembled tubular myelin; (ii) the occasional disappearance of bright spots after bath perfusion, consistent with washout of some surfactant material; and (iii) the identity in size and shape with LTG-stained vesicles. This does not prove that lipids are exclusively stained by FM 1–43, because LB also contain an amorphous material, most likely proteins (31). It is unlikely, however, that LB matrix staining by FM 1–43 contributes significantly to the bright LB fluorescence because of the biophysical properties of FM 1–43, the fact that the core of LB is darker than the periphery (Fig. 1E), and because stained LB structures are destroyed by detergens (unpublished observation). Hence, we suggest that FM 1–43 is an excellent tool to specifically track surfactant material after exocytosis, offering not only the advantage to indicate time of exocytosis (i.e., the onset of fluorescence increase) and cellular location, but also the amount and fate of exocytosed material in the extracellular space. Compared with the staining method by LTG, the high signal-to-background ratio of FM 1–43 (compare with Figs. 1 and 4) in combination with its fast insertion into already exocytosed material (compare washout procedure of Fig. 6) yield a better resolution with respect to onset and quantity of exocytosis. The persistence of discrete spherical fluorescent spots after exocytosis indicates that surfactant material remains aggregated and does not readily dissolve in a physiological bath solution. It is obvious that this portion of surfactant would not be accessible to measurements of secreted phospholipid into the bath, as long as it remains firmly attached to the cells. In fact, the inability to remove cell-attached material even with high perfusion rates (Fig. 6) suggests that exocytosed surfactant is not readily released from the cells, and in some instances it appeared that it might even be retrieved back to the interior of the cell. It is therefore conceivable that the assessment of secreted phospholipids in cell supernatants may have the tendency to underestimate true exocytotic activity, particularly during early stimulation. Accordingly, it is possible that the solubility of surfactant in the aqueous hypophase may be a limiting factor for replenishing the surface film in the native alveolus, because undissolved vesicular forms of surfactant indeed are found in this compartment.
[Ca2+]i and Exocytosis.
A steep dependence of exocytosis on [Ca2+]i is common to various secretory cell types. In the synaptic nerve terminal, Ca2+ spikes in the millisecond range trigger the final fusion event of docked vesicles in the presynaptic membrane (35). Unlike synapses, where high, localized Ca2+ transients cause immediate transmitter release, slower forms of secretion in various neuroendocrine cells may be explained by Ca2+ transients of slower time course and smaller peak amplitude (36, 37). The latter is most likely to reflect a spatial separation between the site of Ca2+ flux and the Ca2+-sensing structure associated with secretory vesicles (36). High, local Ca2+ concentrations at the site of vesicle fusion may be achieved either by local release from intracellular stores (38) or by entry through Ca2+ channels in the PM (39). In AT II cells, there is little information about the mechanisms mediating an elevation of [Ca2+]i: L-type voltage-dependent Ca2+ channels are present in an epithelial cell line of pulmonary origin (40, 41), and indirect evidence suggests their presence in AT II cells as well (42), but their physiological significance remains to be established. A role of Ca2+ in surfactant secretion is strongly suggested by the use of Ca2+ ionophores (13, 18). Its relative importance during stimulation with agonists, however, is still a matter of debate. It has been shown in many studies that ATP evokes a high transient elevation of [Ca2+]i as a result of intracellular Ca2+ release (15, 43–45). Nevertheless, Rice et al. doubted if Ca2+ mobilization is a necessary step for ATP-induced surfactant secretion (44), and, in line with this conclusion, Chander reported that the major part of ATP-induced surfactant secretion was mediated via activation of PKC (16). It should be noted, however, that measurements of surfactant secretion were made within several minutes to hours, whereas Ca2+ mobilization occurs immediately after addition of ATP (within seconds). It is well conceivable, therefore, that persistent ATP-induced surfactant secretion does not depend significantly on sustained high [Ca2+]i, but on the action of alternative signaling cascades (see Introduction). In addition, these studies do not rule out a possible PKC function in controlling the size of the “readily releasable pool,” as has been shown for chromaffin cells (46). Here we show that in the majority of responding cells, LB exocytosis essentially coincides with Ca2+ mobilization. This suggests a role of Ca2+ in a final step of LB fusion in fast responders. The delayed exocytosis of other LB in the same cell and in slow responders, however, is likely to reflect additional mechanisms during earlier stages of vesicle processing or transport.
Time Course of Exocytosis.
Our data demonstrate that agonist-induced exocytosis initiates within seconds to minutes, at the latest (Fig. 5), persists with decreasing rate (Fig. 2), and terminates within 1 hr of stimulation (Fig. 2). Hence, this activity is among the slowest known for mammalian cells. A comparable time course is found in pancreatic acinar cells (47) and in mast cells (48). This slow secretory rate is not unexpected considering the inverse relation between the velocity of secretion and vesicle diameter (49). In fact, all these cells have secretory granules in the micrometer range. Thus, even a slow rate of exocytosis accomplishes secretion of relatively large amounts of stored material. In other cell types it was shown that the vesicle size is also an important determinant for the kinetics of the fusion pore opening (49). In beige mice, de Toledo et al. estimated a “food duration” of ≈1 s for a vesicle radius of 1 μm, during which the fusion pore is already formed but in a fluctuating manner, releasing only small amounts of secretory substance (49). During that time, other compounds like quinacrine do not pass the fusion pore unless it is fully expanded (50). Hence, the permeation properties of the early fusion pore might be limiting for the use of fluorescent dyes to resolve the initial event of fusion, which is most precisely detected by cell capacitance measurements. If LTG and FM 1–43 were equally permeant through the fusion pore, both methods would be expected to detect exocytosis at the same time. In fact, we found that the onset of FM 1–43 fluorescence increase slightly preceded the onset of LTG fluorescence decrease. This might be explained by a better ability of FM 1–43 than LTG to pass the fusion pore owing to its amphiphilic properties. The delay between the peak [Ca2+]i and the onset of FM 1–43 increase of our study could, however, still be an overestimate of the true time lag between stimulus and pore formation. These initial events of exocytosis are, however, certainly not rate limiting for the secretion of surfactant, because this material is hydrophobic and stored as a complex, supramolecular structure in LB. This is supported by our observation that bright FM 1–43-stained spots remained at the site of exocytosis and did not spread into the surrounding medium. Furthermore, the observation that some exocytosed LB were retrieved from the cell periphery to the perinuclear region corresponds to translocation processes of endocytosed material (51) and suggests transient rather than total fusion events, as already described for other cells (49, 50, 52, 53).
Exocytosis is a fundamental process in cell biology, and its regulation belongs to one of the most intensively studied phenomena. In the nervous system and other secretory cell types, exocytosis is so fast that its velocity is one of the major impediments for the investigation of the mechanisms involved therein. In our study we show that stimulated exocytosis in AT II cells occurs within seconds to minutes as compared with milliseconds in other cells. Owing to these kinetic features and the remarkable size of the secretory vesicles enabling direct observation with standard microscopy, this cell type has some major advantages for defining single steps and their sequence in the exocytotic pathway. In the field of pulmonary medicine, the good time resolution of the FM 1–43 method should greatly facilitate the study of repetitiveness of AT II cell stimulation and thus the development of future therapies to enhance surfactant release.
Acknowledgments
We thank Drs. H. Oberleithner and H. Wirtz for valuable discussions, Dr. H. Wirtz for his help setting up the AT II cell preparation, Dr. W. Streif for providing bovine surfactant, and G. Siber, I. Öttl, and H. Heitzenberger for skillful technical assistance. This work was supported by the Fonds zur Förderung der Wissenschaftlichen Forschung, Grants P-11533 and P-10963.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: LB, lamellar body; AT II cell, alveolar type II cell; PM, plasma membrane; LTG, LysoTracker Green DND-26; [Ca2+]i, cytoplasmic Ca2+ concentration; PKC, protein kinase C.
References
- 1.Bennet M K, Scheller R H. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferro-Novick S, Jahn R. Nature (London) 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- 3.Mason, R. J. & Williams, M. C. (1977) Am. Rev. Resp. Dis. 115 (Suppl.), 81–92. [DOI] [PubMed]
- 4.Jarstrand C. In: Pulmonary surfactant. Robertson B, van Golde M L G, Batenburg J J, editors. Amsterdam: Elsevier Science; 1984. pp. 187–201. [Google Scholar]
- 5.Henry J N. J Trauma. 1968;8:756–773. [PubMed] [Google Scholar]
- 6.Coultas P G, Ahier R G, Anderson R L. J Rad Oncol Biol Phys. 1987;13:233–237. doi: 10.1016/0360-3016(87)90132-5. [DOI] [PubMed] [Google Scholar]
- 7.Hollingsworth M, Gilfillan A M. Pharmacol Rev. 1984;36:69–90. [PubMed] [Google Scholar]
- 8.Chander A, Fisher A B. Am J Physiol. 1990;258:L241–L253. doi: 10.1152/ajplung.1990.258.6.L241. [DOI] [PubMed] [Google Scholar]
- 9.Rooney S A, Young S L, Mendelson C R. FASEB J. 1994;8:957–967. doi: 10.1096/fasebj.8.12.8088461. [DOI] [PubMed] [Google Scholar]
- 10.Mason R J, Shannon J M. In: The Lung: Scientific Foundations Second Edition. Crystal R G, West J B, editors. Philadelphia: Lippincott–Raven; 1997. pp. 543–555. [Google Scholar]
- 11.Wirtz H R, Dobbs L G. Science. 1990;250:1266–1269. doi: 10.1126/science.2173861. [DOI] [PubMed] [Google Scholar]
- 12.Mettler N R, Gray M E, Schuffman S, LeQuire V S. Lab Invest. 1981;45:575–586. [PubMed] [Google Scholar]
- 13.Sano K, Voelker D R, Mason R J. Am J Physiol. 1987;253:C679–C686. doi: 10.1152/ajpcell.1987.253.5.C679. [DOI] [PubMed] [Google Scholar]
- 14.Warburton D, Buckley S, Cosico L. J Appl Physiol. 1989;66:901–905. doi: 10.1152/jappl.1989.66.2.901. [DOI] [PubMed] [Google Scholar]
- 15.Rice W R, Singleton F M. Br J Pharmacol. 1987;91:833–838. doi: 10.1111/j.1476-5381.1987.tb11282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chander A, Sen N, Wu A-M, Spitzer A R. Am J Physiol. 1995;268:L108–L116. doi: 10.1152/ajplung.1995.268.1.L108. [DOI] [PubMed] [Google Scholar]
- 17.Gobran L I, Xu Z X, Lu Z, Rooney S A. Am J Physiol. 1994;267:L625–L633. doi: 10.1152/ajplung.1994.267.5.L625. [DOI] [PubMed] [Google Scholar]
- 18.Dobbs L G, Gonzales R F, Marinari L A, Mescher E J, Hawgood S. Biochem Biophys Acta. 1986;877:305–313. doi: 10.1016/0005-2760(86)90308-5. [DOI] [PubMed] [Google Scholar]
- 19.Sano K, Voelker D R, Mason R. J Biol Chem. 1985;260:12725–12729. [PubMed] [Google Scholar]
- 20.Tsilibary E C, Williams M C. J Histochem Cytochem. 1983;31:1289–1304. doi: 10.1177/31.11.6684669. [DOI] [PubMed] [Google Scholar]
- 21.Corbet A J, Voelker R M, Murphy F M, Owens M L. J Appl Physiol. 1988;65:1710–1715. doi: 10.1152/jappl.1988.65.4.1710. [DOI] [PubMed] [Google Scholar]
- 22.Rice W R, Osterhoudt K C, Whitsett J A. Biochim Biophys Acta. 1984;805:12–18. doi: 10.1016/0167-4889(84)90030-2. [DOI] [PubMed] [Google Scholar]
- 23.Chander A, Wu R D. Biochim Biophys Acta. 1991;1086:157–166. doi: 10.1016/0005-2760(91)90003-z. [DOI] [PubMed] [Google Scholar]
- 24.Sen N, Spitzer A R, Chander A. Biochem J. 1997;1322:103–109. doi: 10.1042/bj3220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Fisher A B, Zimmerman U-J P. Biochim Biophys Acta. 1995;1259:166–172. doi: 10.1016/0005-2760(95)00159-a. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Wang M, Fisher A B, Zimmerman U-J P. Am J Physiol. 1996;270:L668–L676. doi: 10.1152/ajplung.1996.270.4.L668. [DOI] [PubMed] [Google Scholar]
- 27.Dobbs L G, Gonzalez R, Williams M C. Am Ref Respir Dis. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 28.Haller T, Dietl P, Deetjen P, Völkl H. Cell Calcium. 1986;19:157–165. doi: 10.1016/s0143-4160(96)90084-6. [DOI] [PubMed] [Google Scholar]
- 29.Chander A, Johnson R G, Reicherter J, Fisher A B. J Biol Chem. 1986;261:6126–6131. [PubMed] [Google Scholar]
- 30.Betz W J, Bewick G S. Science. 1992;255:200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- 31.Gil J. Annu Rev Physiol. 1985;47:753–763. doi: 10.1146/annurev.ph.47.030185.003541. [DOI] [PubMed] [Google Scholar]
- 32.Zeuzem S, Feick P, Zimmermann P, Haase W, Kahn R A, Schulz I. Proc Natl Acad Sci USA. 1992;89:6619–6623. doi: 10.1073/pnas.89.14.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weibel E R, Staubli W, Gnagi H R, Hess F A. J Cell Biol. 1969;42:68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith C B, Betz W J. Nature (London) 1996;380:531–534. doi: 10.1038/380531a0. [DOI] [PubMed] [Google Scholar]
- 35.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 36.Chow R H, Klingauf J, Neher E. Proc Natl Acad Sci USA. 1994;91:12765–12769. doi: 10.1073/pnas.91.26.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heidelberger R, Heinemann C, Neher E, Matthews G. Nature (London) 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 38.Tse F W, Tse A, Hille B, Horstmann H, Almers W. Neuron. 1997;18:121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- 39.Robinson I M, Finnegan J M, Monck J R, Wightman R M, Fernandez J M. Proc Natl Acad Sci USA. 1995;92:2474–2478. doi: 10.1073/pnas.92.7.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietl P, Haller T, Wirleitner B, Völkl H, Friedrich F, Striessnig J. Am J Physiol. 1995;269:L873–L883. doi: 10.1152/ajplung.1995.269.6.L873. [DOI] [PubMed] [Google Scholar]
- 41.Schobersberger W, Friedrich F, Hoffmann G, Völkl H, Dietl P. Am J Physiol. 1997;272:L1092–L1097. doi: 10.1152/ajplung.1997.272.6.L1092. [DOI] [PubMed] [Google Scholar]
- 42.Sen N, Grunstein M M, Chander A. Am J Physiol. 1994;266:L255–L262. doi: 10.1152/ajplung.1994.266.3.L255. [DOI] [PubMed] [Google Scholar]
- 43.Dorn C C, Rice W R, Singleton F M. Br J Pharmacol. 1989;97:163–170. doi: 10.1111/j.1476-5381.1989.tb11938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice W R, Dorn C C, Singleton F M. Biochem J. 1990;266:407–413. doi: 10.1042/bj2660407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rooney S A, Gobran L I, Griese M. Prog Respir Res. 1994;27:84–91. [Google Scholar]
- 46.Gillis K D, Mössner R, Neher E. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 47.Schneider S W, Sritharan K C, Geibel J P, Oberleithner H, Jena B P. Proc Natl Acad Sci USA. 1997;94:316–321. doi: 10.1073/pnas.94.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almers W, Neher E. J Physiol. 1987;386:205–217. doi: 10.1113/jphysiol.1987.sp016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez de Toledo G, Fernandez-Chacon R, Fernandez J M. Nature (London) 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 50.Beckenridge L J, Almers W. Proc Natl Acad Sci USA. 1987;84:1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aniento F, Emans N, Griffith G, Gruenberg J. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jena B P. Cell Biol Int. 1997;21:257–259. doi: 10.1006/cbir.1997.0159. [DOI] [PubMed] [Google Scholar]
- 53.Maruyama Y, Petersen O H. Cell Calcium. 1994;16:419–430. doi: 10.1016/0143-4160(94)90035-3. [DOI] [PubMed] [Google Scholar]