Abstract
In the CNS, nitric oxide (NO) has been implicated as both a mediator of neurotoxicity and a neuromodulator. The inducible NO synthase (iNOS), thought to mediate toxic effects of NO, has been attributed to glial cells in the CNS. We now report that cerebellar granule cell neurones can be stimulated by lipopolysaccharide and interferon-gamma to express iNOS in vitro, as demonstrated by reverse transcription-polymerase chain reaction and fluorescent in situ hybridisation. The expression of both constitutive NO synthase (cNOS) and iNOS by neurones suggests that NO has diverse functions in the brain, and supports the possibility that iNOS plays a role in neuronal damage and inflammation following activation of brain microglia and production of cytokines.
Full text
PDF
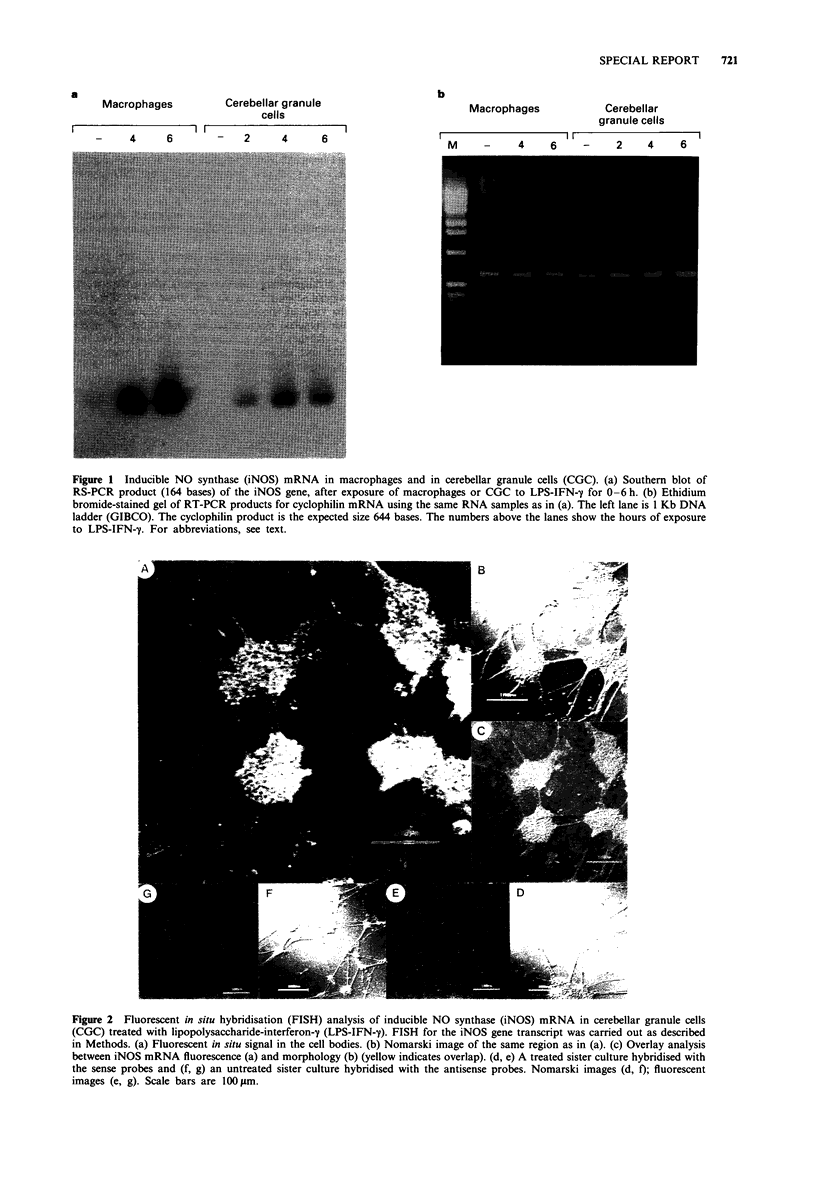

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Zheng Y. M., Heber-Katz E., Fraser N., Rorke L., Fu Z. F., Hanlon C., Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morganti-Kossmann M. C., Kossmann T., Wahl S. M. Cytokines and neuropathology. Trends Pharmacol Sci. 1992 Jul;13(7):286–291. doi: 10.1016/0165-6147(92)90087-m. [DOI] [PubMed] [Google Scholar]
- Murphy S., Simmons M. L., Agullo L., Garcia A., Feinstein D. L., Galea E., Reis D. J., Minc-Golomb D., Schwartz J. P. Synthesis of nitric oxide in CNS glial cells. Trends Neurosci. 1993 Aug;16(8):323–328. doi: 10.1016/0166-2236(93)90109-y. [DOI] [PubMed] [Google Scholar]
- Novelli A., Reilly J. A., Lysko P. G., Henneberry R. C. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988 Jun 7;451(1-2):205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Nowicki J. P., Duval D., Poignet H., Scatton B. Nitric oxide mediates neuronal death after focal cerebral ischemia in the mouse. Eur J Pharmacol. 1991 Nov 12;204(3):339–340. doi: 10.1016/0014-2999(91)90862-k. [DOI] [PubMed] [Google Scholar]
- Shuldiner A. R., Tanner K., Moore C. A., Roth J. RNA template-specific PCR: an improved method that dramatically reduces false positives in RT-PCR. Biotechniques. 1991 Dec;11(6):760–763. [PubMed] [Google Scholar]
- Tsarfaty I., Resau J. H., Rulong S., Keydar I., Faletto D. L., Vande Woude G. F. The met proto-oncogene receptor and lumen formation. Science. 1992 Aug 28;257(5074):1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]




