Abstract
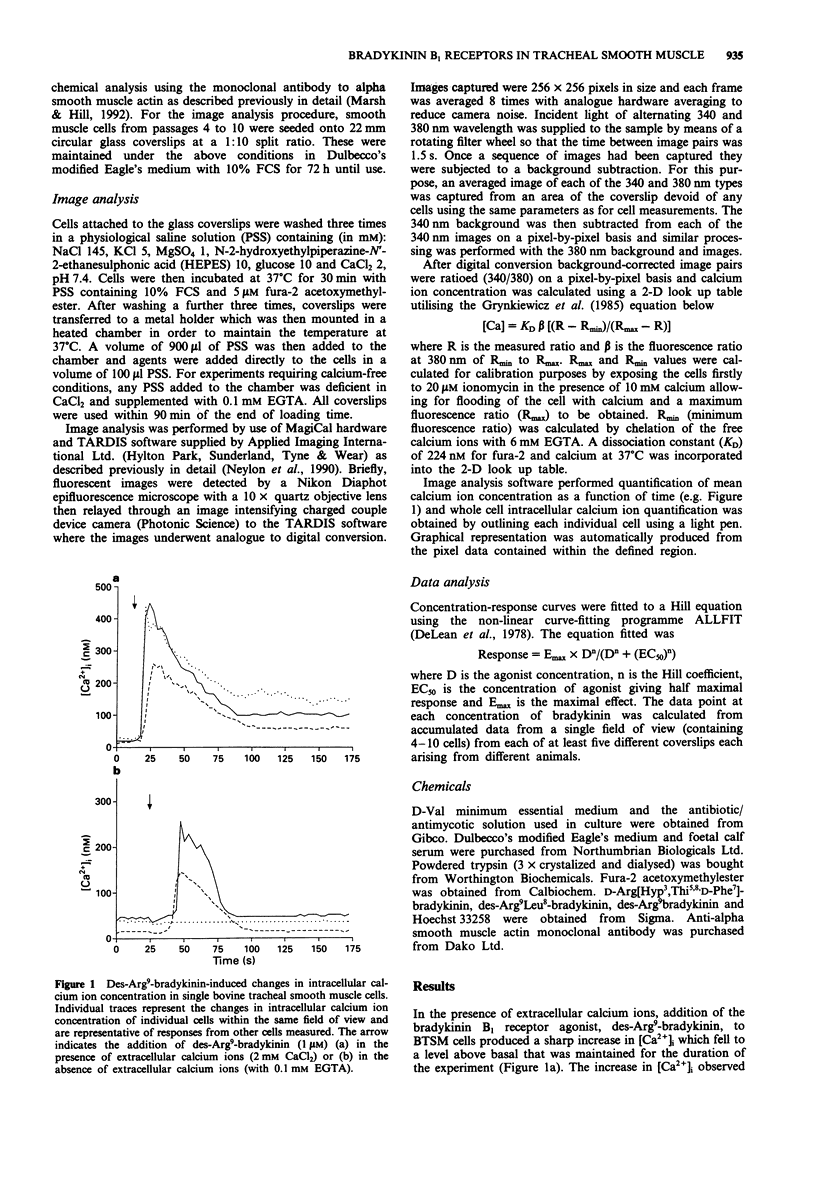
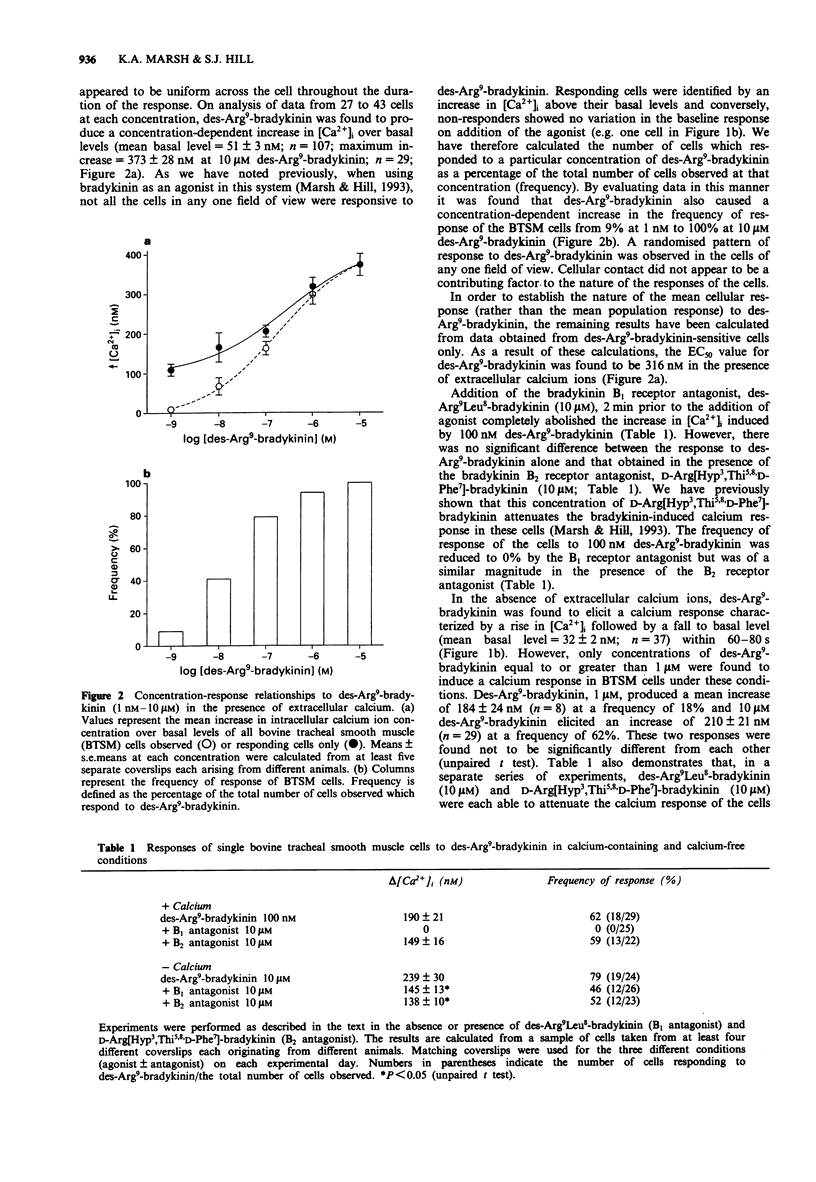
1. Dynamic video imaging was used to measure the des-Arg9-bradykinin-induced changes in the intracellular free calcium ion concentration ([Ca2+]i) of single bovine tracheal smooth muscle (BTSM) cells. 2. In the presence of extracellular calcium ions, des-Arg9-bradykinin (1 nM-10 microM) produced a concentration-dependent increase in the [Ca2+]i over basal levels yielding an EC50 value of 316 nM. The percentage of cells responding to each concentration of des-Arg9-bradykinin also increased in a concentration-dependent manner (from 9% to 100%). 3. The bradykinin B2 receptor antagonist, D-Arg[Hyp3,Thi5,8,D-Phe7]-bradykinin (10 microM), was without effect on the calcium response of the cells when added 2 min prior to des-Arg9-bradykinin (100 nM). However, the B1 receptor antagonist, des-Arg9Leu8-bradykinin (10 microM), completely abolished the des-Arg9-bradykinin-induced response. 4. Under calcium-free conditions, des-Arg9-bradykinin induced an increase in [Ca2+]i at concentrations of 1 microM and 10 microM. The response to 10 microM des-Arg9-bradykinin was reduced by preincubation of either D-Arg[Hyp3, Thi5,8,D-Phe7]-bradykinin (10 microM) or des-Arg9Leu8-bradykinin (10 microM). 5. We conclude that bradykinin B1 receptors are expressed by cultured BTSM cells and mediate the des-Arg9-bradykinin-induced influx of calcium ions at low agonist concentrations (< 1 microM). At higher concentrations, des-Arg9-bradykinin (1 microM and 10 microM) can stimulate both B1 and B2 receptors to effect intracellular calcium release under calcium-free conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascands J. L., Pecher C., Rouaud S., Emond C., Tack J. L., Bastie M. J., Burch R., Regoli D., Girolami J. P. Evidence for existence of two distinct bradykinin receptors on rat mesangial cells. Am J Physiol. 1993 Mar;264(3 Pt 2):F548–F556. doi: 10.1152/ajprenal.1993.264.3.F548. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Christiansen S. C., Proud D., Cochrane C. G. Detection of tissue kallikrein in the bronchoalveolar lavage fluid of asthmatic subjects. J Clin Invest. 1987 Jan;79(1):188–197. doi: 10.1172/JCI112782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., Burch R. M., Kyle D. J., Martin J. A., Meeker S. N., Togo J. D-Arg[Hyp3-Thi5-D-Tic7-Tic8]-bradykinin, a potent antagonist of smooth muscle BK2 receptors and BK3 receptors. Br J Pharmacol. 1991 Apr;102(4):785–787. doi: 10.1111/j.1476-5381.1991.tb12251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. G., Burch R. M., Meeker S. A., Wilkins D. E. Evidence for a pulmonary B3 bradykinin receptor. Mol Pharmacol. 1989 Jul;36(1):1–8. [PubMed] [Google Scholar]
- Farmer S. G., Ensor J. E., Burch R. M. Evidence that cultured airway smooth muscle cells contain bradykinin B2 and B3 receptors. Am J Respir Cell Mol Biol. 1991 Mar;4(3):273–277. doi: 10.1165/ajrcmb/4.3.273. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Marsh K. A., Hill S. J. Bradykinin B2 receptor-mediated phosphoinositide hydrolysis in bovine cultured tracheal smooth muscle cells. Br J Pharmacol. 1992 Oct;107(2):443–447. doi: 10.1111/j.1476-5381.1992.tb12765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K. A., Hill S. J. Characteristics of the bradykinin-induced changes in intracellular calcium ion concentration of single bovine tracheal smooth muscle cells. Br J Pharmacol. 1993 Sep;110(1):29–35. doi: 10.1111/j.1476-5381.1993.tb13767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre P., Phillips E., Skidmore E., Brown M., Webb M. Cloned murine bradykinin receptor exhibits a mixed B1 and B2 pharmacological selectivity. Mol Pharmacol. 1993 Aug;44(2):346–355. [PubMed] [Google Scholar]
- Murray R. K., Kotlikoff M. I. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991 Apr;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon C. B., Hoyland J., Mason W. T., Irvine R. F. Spatial dynamics of intracellular calcium in agonist-stimulated vascular smooth muscle cells. Am J Physiol. 1990 Oct;259(4 Pt 1):C675–C686. doi: 10.1152/ajpcell.1990.259.4.C675. [DOI] [PubMed] [Google Scholar]
- Regoli D. C., Marceau F., Lavigne J. Induction of beta 1-receptors for kinins in the rabbit by a bacterial lipopolysaccharide. Eur J Pharmacol. 1981 Apr 24;71(1):105–115. doi: 10.1016/0014-2999(81)90391-5. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Regoli D., Rhaleb N. E., Drapeau G., Dion S. Kinin receptor subtypes. J Cardiovasc Pharmacol. 1990;15 (Suppl 6):S30–S38. [PubMed] [Google Scholar]
- Somlyo A. P., Walker J. W., Goldman Y. E., Trentham D. R., Kobayashi S., Kitazawa T., Somlyo A. V. Inositol trisphosphate, calcium and muscle contraction. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):399–414. doi: 10.1098/rstb.1988.0084. [DOI] [PubMed] [Google Scholar]


