Abstract
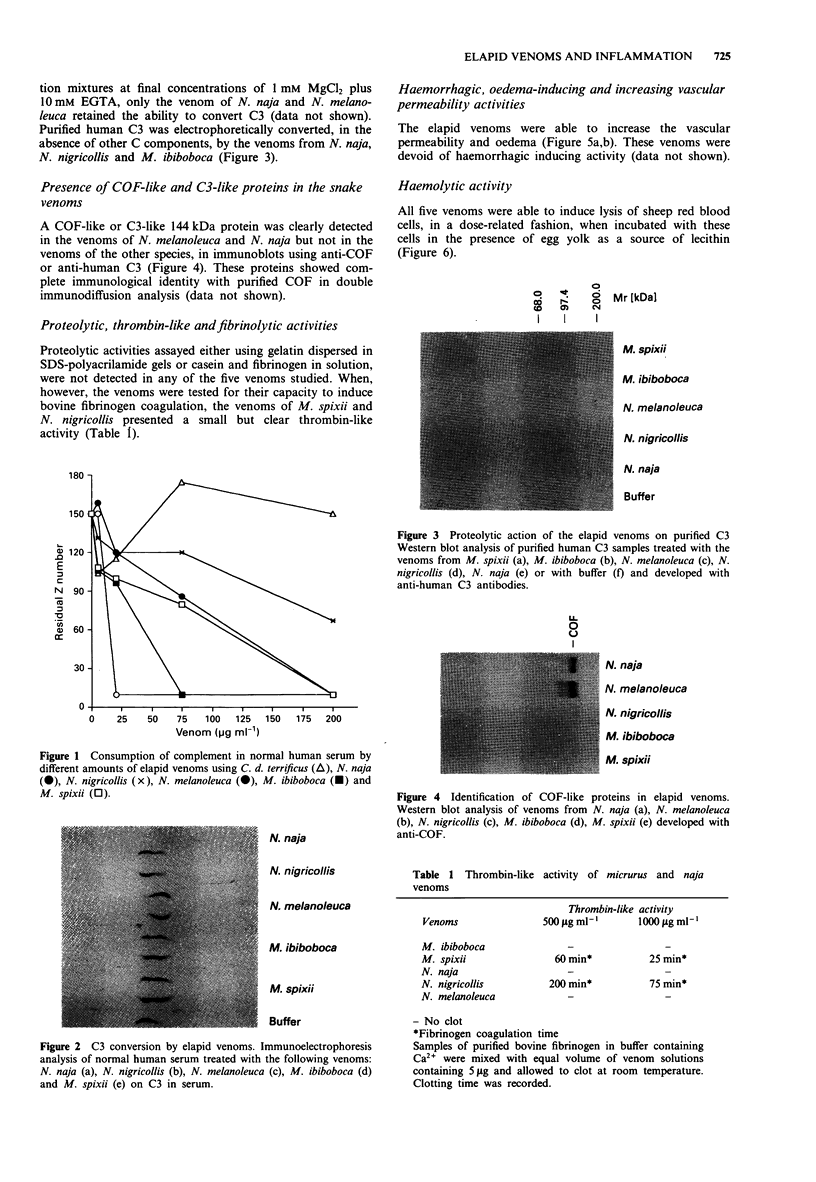
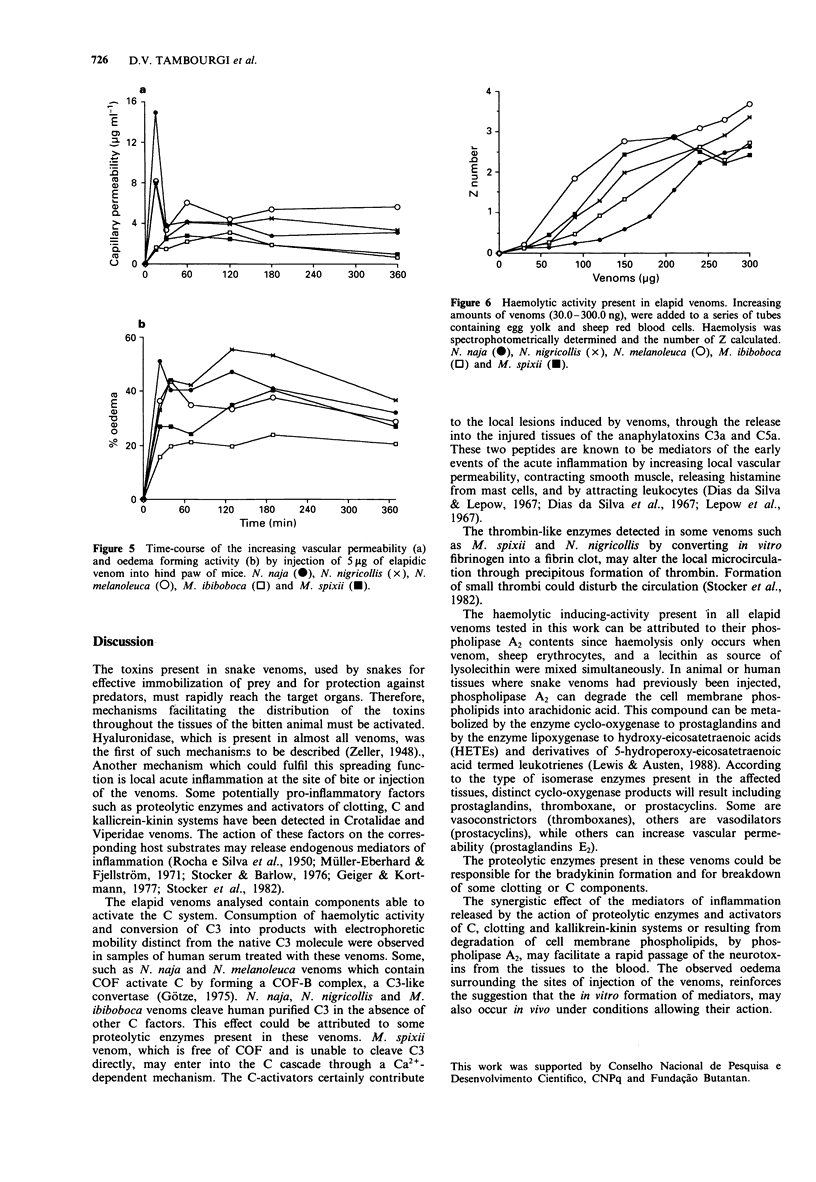
1. Snake venoms from the genera Micrurus (M. ibiboboca and M. spixii) and Naja (N. naja, N. melanoleuca and N. nigricollis) were analysed, using biological and immunochemical methods, to detect pro-inflammatory activities, cobra venom factor (COF), proteolytic enzymes, thrombin-like substances, haemorrhagic and oedema-producing substances. 2. The venoms of the five snake species activate the complement system (C) in normal human serum (NHS) in a dose-related fashion, at concentrations ranging from 5 micrograms to 200 micrograms ml-1 serum. Electrophoretic conversion of C3 was observed with all venoms in NHS containing normal concentrations of Ca2+ and Mg2+, but only by venoms from N. naja and N. melanoleuca when Ca2+ was chelated by adding Mg(2+)-EGTA. 3. Purified human C3 was electrophoretically converted, in the absence of other C components, by the venoms from N. naja, N. nigricollis and M. ibiboboca. However, only the venoms from N. naja and N. melanoleuca contained a 144 kDa protein revealed in Western blot with sera against COF or human C3. 4. All venoms, at minimum concentrations of 30 ng ml-1, were capable of lysing sheep red blood cells, also in a dose-related fashion, when incubated with these cells in presence of egg yolk as a source of lecithin. Although the venoms from M. spixii and N. nigricollis showed detectable thrombin-like activity, these and the other venoms were free of proteolytic activity when fibrin, gelatin and casein, were used as substrates. 5. When tested on mice skin, all five venoms were capable of inducing an increase in vascular permeability and oedema, but were devoid of haemorrhagic producing substances (haemorrhagins).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Balavitch D. Cobra venom factor: evidence for its being altered cobra C3 (the third component of complement). Science. 1976 Mar 26;191(4233):1275–1276. doi: 10.1126/science.56780. [DOI] [PubMed] [Google Scholar]
- Dias Da Silva W., Lepow I. H. Complement as a mediator of inflammation. II. Biological properties of anaphylatoxin prepared with purified components of human complement. J Exp Med. 1967 May 1;125(5):921–946. doi: 10.1084/jem.125.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsen G., Lundwall A., Hellman U., Sjöquist J. Antigenic relationships between human and cobra complement factors C3 and cobra venom factor (CVF) from the Indian cobra (Naja naja). J Immunol. 1983 Oct;131(4):1920–1923. [PubMed] [Google Scholar]
- Geiger R., Kortmann H. Esterolytic and proteolytic activities of snake venoms and their inhibition by proteinase inhibitors. Toxicon. 1977;15(3):257–259. doi: 10.1016/0041-0101(77)90052-6. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- KONDO H., KONDO S., IKEZAWA H., MURATA R. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn J Med Sci Biol. 1960;13:43–52. doi: 10.7883/yoken1952.13.43. [DOI] [PubMed] [Google Scholar]
- Leme J. G., Wilhelm D. L. The effects of adrenalectomy and corticosterone on vascular permeability responses in the skin of the rat. Br J Exp Pathol. 1975 Oct;56(5):402–407. [PMC free article] [PubMed] [Google Scholar]
- Lomonte B., Gutiérrez J. M. La actividad proteolítica de los venenos de serpientes de Costa Rica sobre la caseína. Rev Biol Trop. 1983 Jun;31(1):37–40. [PubMed] [Google Scholar]
- Mandelbaum F. R., Assakura M. T. Antigenic relationship of hemorrhagic factors and proteases isolated from the venoms of three species of Bothrops snakes. Toxicon. 1988;26(4):379–385. doi: 10.1016/0041-0101(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Fjellström K. E. Isolation of the anticomplementary protein from cobra venom and its mode of action on C3. J Immunol. 1971 Dec;107(6):1666–1672. [PubMed] [Google Scholar]
- Pickering R. J., Wolfson M. R., Good R. A., Gewurz H. Passive hemolysis by serum and cobra venom factor: a new mechanism inducing membrane damage by complement. Proc Natl Acad Sci U S A. 1969 Feb;62(2):521–527. doi: 10.1073/pnas.62.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Medof M. E. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–267. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Gewurz H., Snyderman R. Reaction of a cobra venom factor with guinea pig complement and generation of an activity chemotactic for polymorphonuclear leukocytes. Proc Soc Exp Biol Med. 1969 May;131(1):203–207. doi: 10.3181/00379727-131-33840. [DOI] [PubMed] [Google Scholar]
- Stocker K., Barlow G. H. The coagulant enzyme from Bothrops atrox venom (batroxobin). Methods Enzymol. 1976;45:214–223. doi: 10.1016/s0076-6879(76)45021-8. [DOI] [PubMed] [Google Scholar]
- Stocker K., Fischer H., Meier J. Thrombin-like snake venom proteinases. Toxicon. 1982;20(1):265–273. doi: 10.1016/0041-0101(82)90225-2. [DOI] [PubMed] [Google Scholar]
- da Silva W. D., Eisele J. W., Lepow I. H. Complement as a mediator of inflammation. 3. Purification of the activity with anaphylatoxin properties generated by interaction of the first four components of complement and its identification as a cleavage product of C'3. J Exp Med. 1967 Dec 1;126(6):1027–1048. doi: 10.1084/jem.126.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]





