Abstract
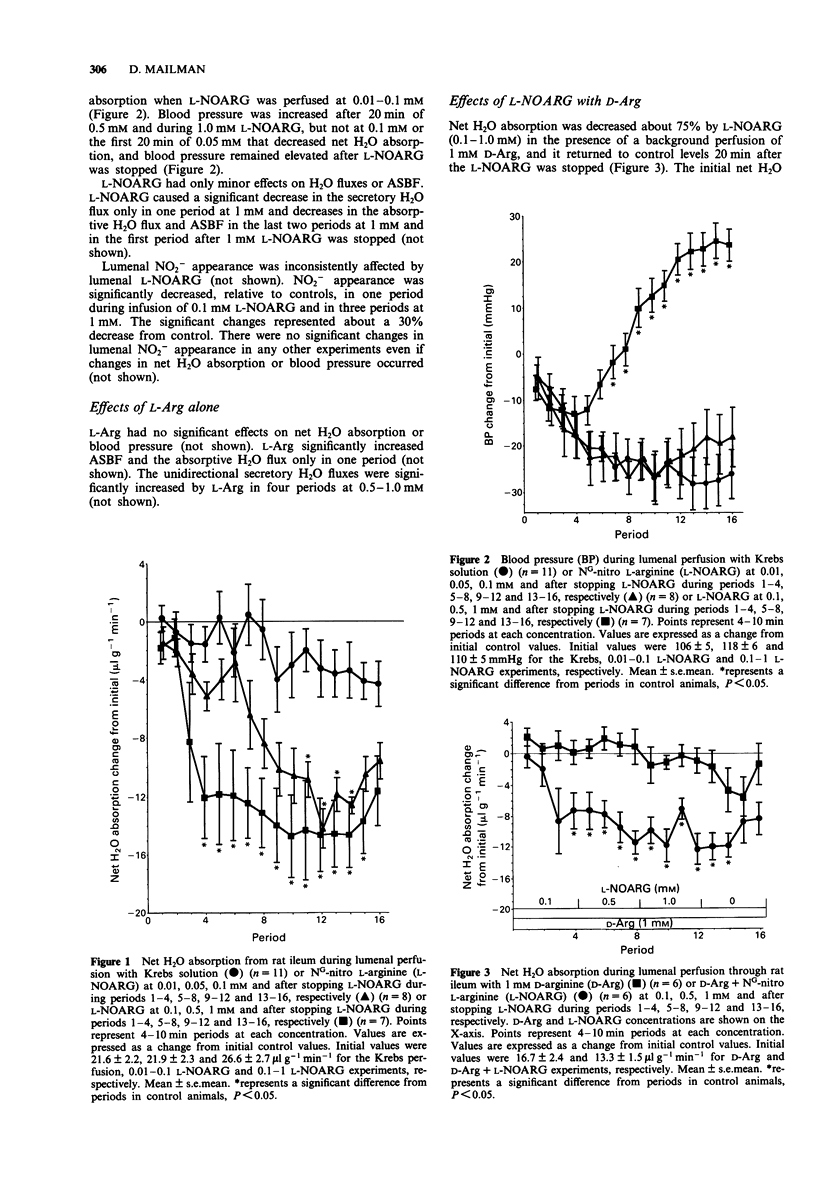
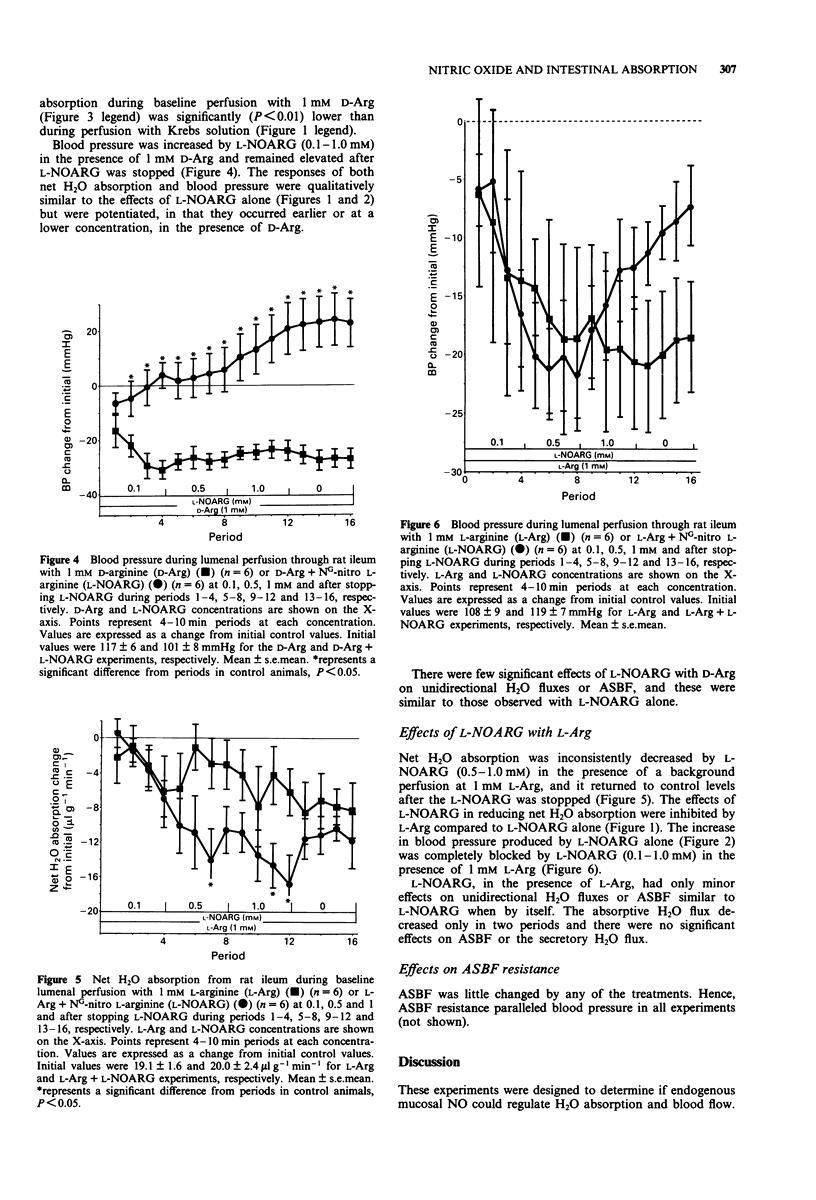
1. The role of endogenous mucosal nitric oxide (NO) in the local regulation of H2O absorption and blood flow in rat ileum was studied by perfusing L-arginine (L-Arg) (0.1-1.0 mM) and NG-nitro L-arginine (L-NOARG) (0.01-1.0 mM) through the lumen. D-Arginine (D-Arg) or L-Arg (1 mM), combined with L-NOARG, were used to determine if any of the measured intestinal effects of L-NOARG were exerted through NO formation. 2. Net and unidirectional H2O fluxes and effective mucosal blood flow were measured using 3H2O and [14C]-inulin in the perfusate. Mucosal NO formation was measured as the appearance of lumenal NO2-. 3. L-NOARG, beginning at a concentration of 0.1 mM, decreased net H2O absorption, but had only minor effects on unidirectional H2O fluxes or on blood flow. L-NOARG increased blood pressure, beginning at a concentration of 0.5 mM. 4. L-Arg had no significant effects on net H2O absorption or blood pressure, and only minor effects on unidirectional H2O fluxes and blood flow. 5. NO appearance in the lumen was marginally decreased by 1.0 mM L-NOARG, but not increased by L-Arg. 6. Mucosal blood flow resistance paralleled systemic blood pressure suggesting that vascular effects on the mucosa were exerted only after L-NOARG had reached the general circulation. 7. Lumenal L-Arg reversed the effects of lumenal L-NOARG on net H2O absorption and blood pressure, but D-Arg did not.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres H., Rock R., Bridges R. J., Rummel W., Schreiner J. Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol. 1985 Jul;364:301–312. doi: 10.1113/jphysiol.1985.sp015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriantsitohaina R., Surprenant A. Acetylcholine released from guinea-pig submucosal neurones dilates arterioles by releasing nitric oxide from endothelium. J Physiol. 1992;453:493–502. doi: 10.1113/jphysiol.1992.sp019241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berschneider H. M., Powell D. W. Fibroblasts modulate intestinal secretory responses to inflammatory mediators. J Clin Invest. 1992 Feb;89(2):484–489. doi: 10.1172/JCI115610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F., M'Rabet-Touil H., Darcy-Vrillon B., Posho L., Duee P. H. Stimulation by D-glucose of the direct conversion of arginine to citrulline in enterocytes isolated from pig jejunum. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1171–1177. doi: 10.1016/0006-291x(91)90663-r. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Ruytjens I. F., Bult H., De Man J. G., Herman A. G., Van Maercke Y. M. Bioassay of nitric oxide released upon stimulation of non-adrenergic non-cholinergic nerves in the canine ileocolonic junction. Br J Pharmacol. 1991 May;103(1):1085–1091. doi: 10.1111/j.1476-5381.1991.tb12304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogle R. G., Moncada S., Pearson J. D., Mann G. E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br J Pharmacol. 1992 Apr;105(4):768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. Role of intestinal basolateral membrane in absorption of nutrients. Am J Physiol. 1992 Sep;263(3 Pt 2):R482–R488. doi: 10.1152/ajpregu.1992.263.3.R482. [DOI] [PubMed] [Google Scholar]
- Cooke H. J. Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol. 1984 Mar;246(3 Pt 1):G263–G267. doi: 10.1152/ajpgi.1984.246.3.G263. [DOI] [PubMed] [Google Scholar]
- Creager M. A., Gallagher S. J., Girerd X. J., Coleman S. M., Dzau V. J., Cooke J. P. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992 Oct;90(4):1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Regional haemodynamic changes during oral ingestion of NG-monomethyl-L-arginine or NG-nitro-L-arginine methyl ester in conscious Brattleboro rats. Br J Pharmacol. 1990 Sep;101(1):10–12. doi: 10.1111/j.1476-5381.1990.tb12079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger J. P., Alberola A. M., Salazar F. J., Nakamura T. Control of renal hemodynamics during intrarenal and systemic blockade of nitric oxide synthesis in conscious dogs. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S160–S162. doi: 10.1097/00005344-199204002-00045. [DOI] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Lefebvre R. A., De Vriese A., Smits G. J. Influence of vasoactive intestinal polypeptide and NG-nitro-L-arginine methyl ester on cholinergic neurotransmission in the rat gastric fundus. Eur J Pharmacol. 1992 Oct 20;221(2-3):235–242. doi: 10.1016/0014-2999(92)90707-b. [DOI] [PubMed] [Google Scholar]
- Lopez-Belmonte J., Whittle B. J., Moncada S. The actions of nitric oxide donors in the prevention or induction of injury to the rat gastric mucosa. Br J Pharmacol. 1993 Jan;108(1):73–78. doi: 10.1111/j.1476-5381.1993.tb13442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman D. Cardiovascular and flux relationships in canine ileum. Am J Physiol. 1984 Oct;247(4 Pt 1):G357–G365. doi: 10.1152/ajpgi.1984.247.4.G357. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh O., Kudo Y., Shikata H., Yamada K., Kawasaki T. Characterization of amino-acid transport systems in guinea-pig intestinal brush-border membrane. Biochim Biophys Acta. 1989 Oct 16;985(2):120–126. doi: 10.1016/0005-2736(89)90355-6. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Wennmalm A., Benthin G., Petersson A. S. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. Br J Pharmacol. 1992 Jul;106(3):507–508. doi: 10.1111/j.1476-5381.1992.tb14365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter J. P., Balish E. Distribution and metabolism of ingested NO3- and NO2- in germfree and conventional-flora rats. Appl Environ Microbiol. 1979 Nov;38(5):861–869. doi: 10.1128/aem.38.5.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter J. P., Gatley S. J., Balish E. Distribution of nitrogen-13 from labeled nitrate (13No3-) in humans and rats. Science. 1979 Apr 27;204(4391):411–413. doi: 10.1126/science.441728. [DOI] [PubMed] [Google Scholar]
- Young H. M., Furness J. B., Shuttleworth C. W., Bredt D. S., Snyder S. H. Co-localization of nitric oxide synthase immunoreactivity and NADPH diaphorase staining in neurons of the guinea-pig intestine. Histochemistry. 1992 May;97(4):375–378. doi: 10.1007/BF00270041. [DOI] [PubMed] [Google Scholar]


