Abstract
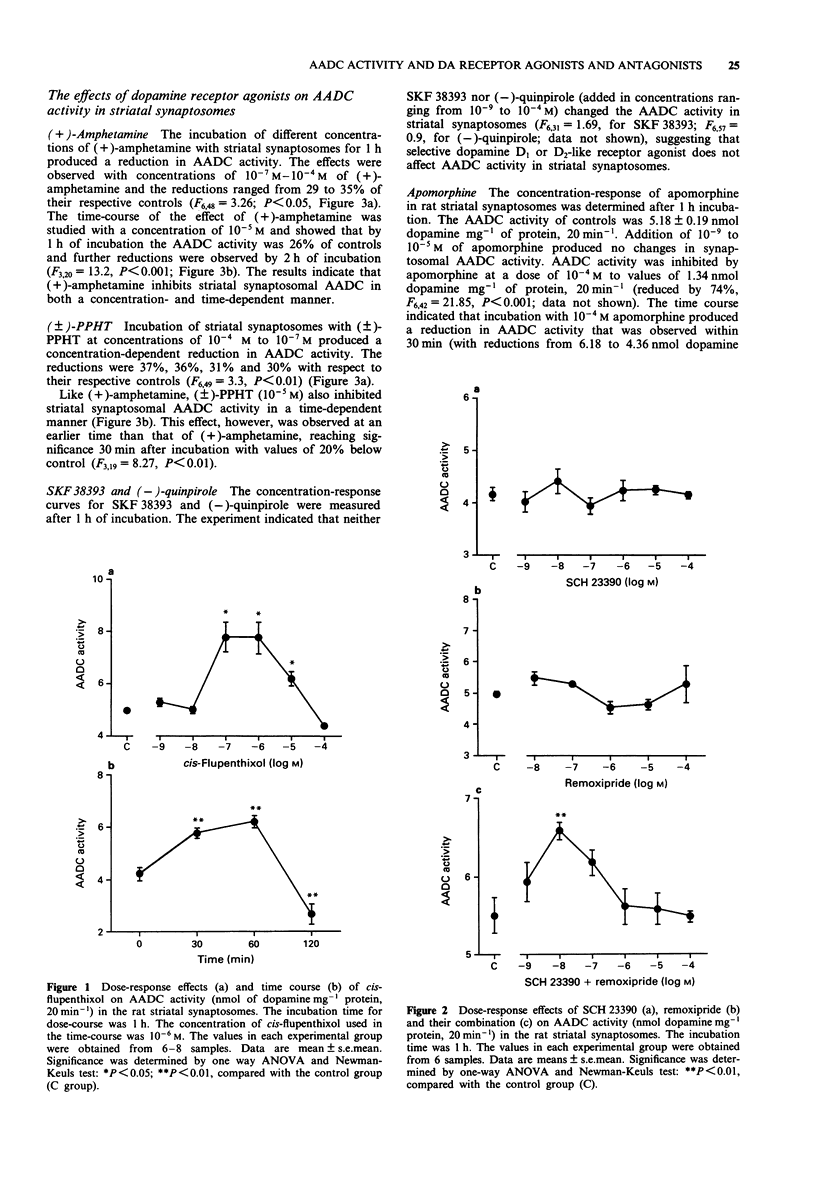
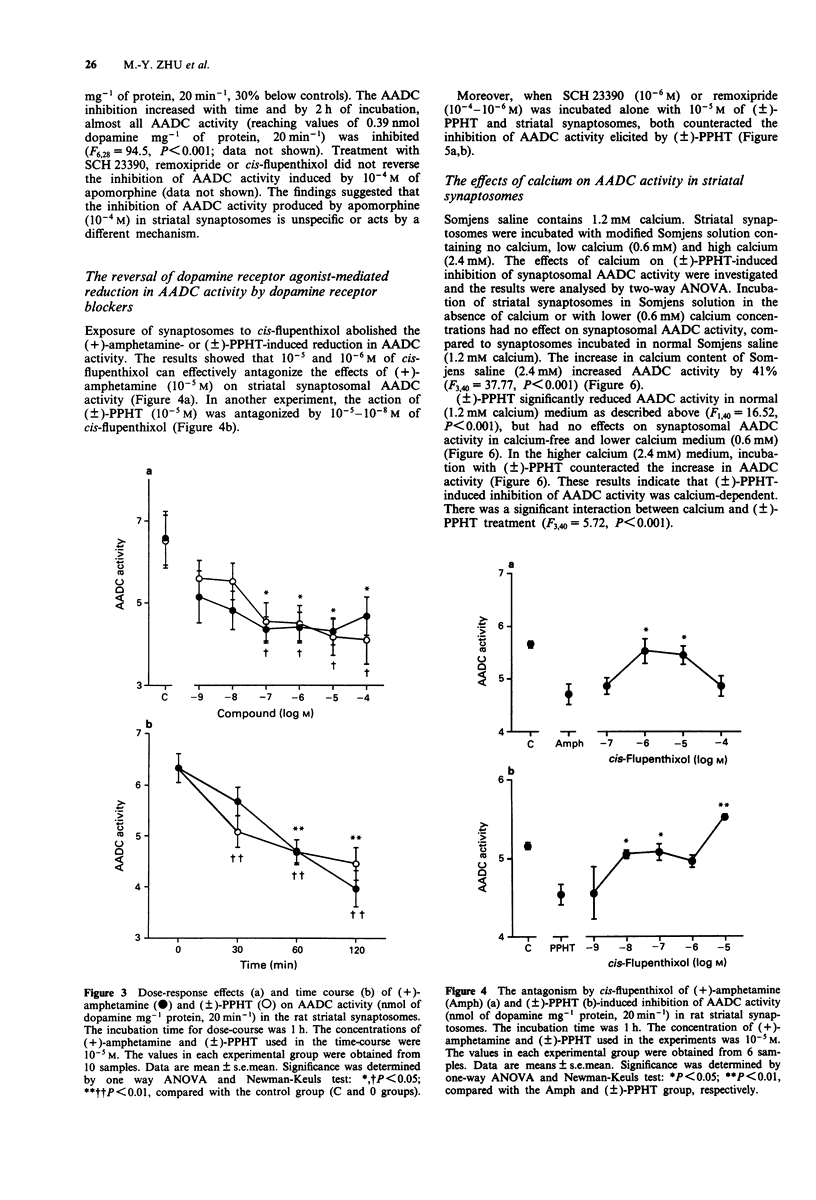
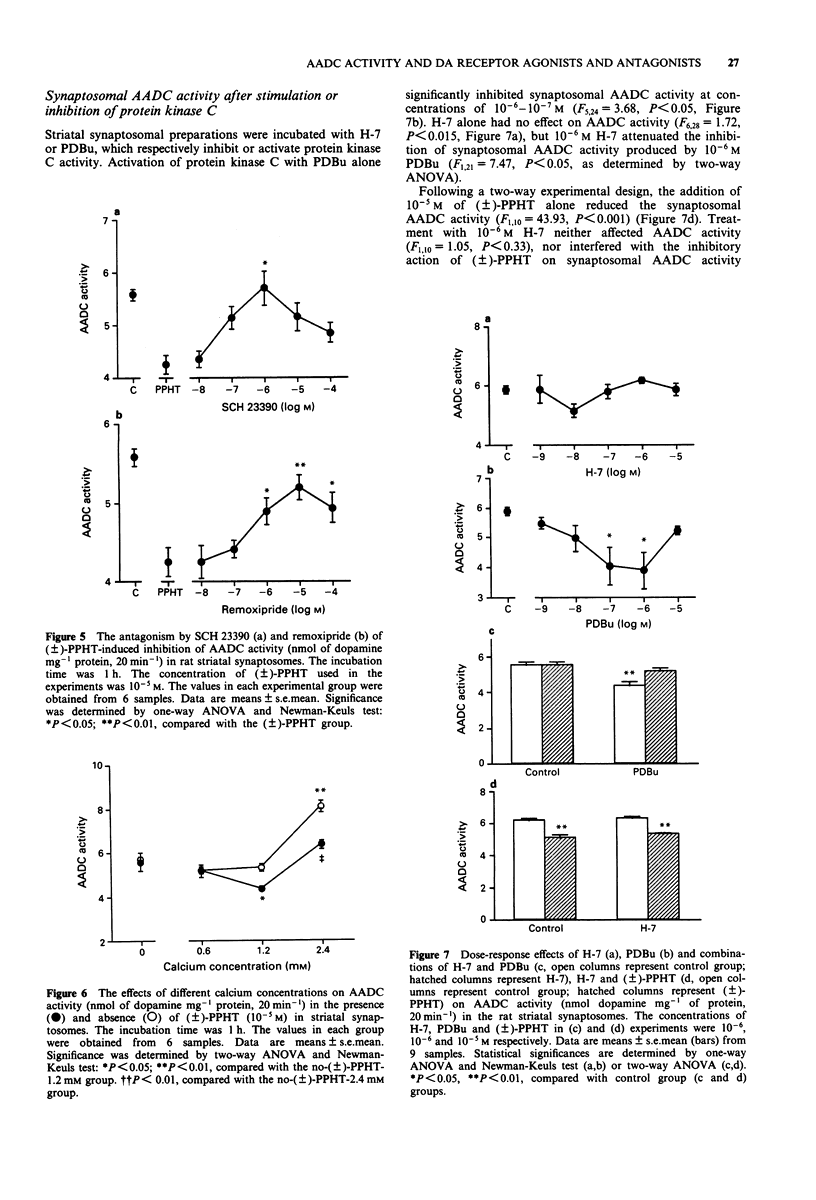
1. In this study we investigated the effects of dopamine receptor agonists and antagonists on rat striatal synaptosomal aromatic L-amino acid decarboxylase (AADC) activity. 2. The results show that 10(-5)-10(-7) M cis-flupenthixol increased the striatal synaptosomal AADC activity (by 25% to 57%) in a time-dependent manner. SCH 23390 and remoxipride alone had little or no effect on striatal synaptosomal AADC activity, but in combination they increased AADC activity by 20%, suggesting that the increases in striatal synaptosomal AADC activity occurred only after blockade of both dopamine D1 and D2 receptors. 3. Treatment with (+)-amphetamine and (+/-)-2-(N-phenylethyl-N-propyl)amino-5- hydroxytetralin hydrochloride ((+/-)-PPHT) produced a reduction of striatal synaptosomal AADC activity in a concentration- and time-dependent manner. SKF 38393 and (-)-quinpirole, however, exhibited no effect on striatal synaptosomal AADC activity, suggesting that only the mixed dopamine receptor agonists can reduce the AADC activity. Incubation with apomorphine at a concentration of 10(-4) M inhibited the AADC activity by 74% and this inhibition cannot be antagonized by SCH 23390, remoxipride or cis-flupenthixol, suggesting that apomorphine-induced inhibition of striatal synaptosomal AADC activity was not mediated by dopamine receptors. 4. cis-Flupenthixol can reverse the reduction of AADC activity induced by (+)-amphetamine and (+/-)-PPHT. The inhibition of AADC activity elicited by (+/-)-PPHT also can be reversed by SCH 23390 and remoxipride. 5. The inhibition of striatal synaptosomal AADC activity induced by (+/-)-PPHT is calcium-dependent and protein kinase C may play a role in the regulation of striatal AADC activity.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitran M., Bustos G. On the mechanism of presynaptic autoreceptor-mediated inhibition of transmitter synthesis in dopaminergic nerve terminals. Biochem Pharmacol. 1982 Sep 15;31(18):2851–2860. doi: 10.1016/0006-2952(82)90254-4. [DOI] [PubMed] [Google Scholar]
- Bräutigam M., Dreesen R., Herken H. Dopa-release from mouse neuroblastoma clone N 1 E-115 into the culture medium. A test for tyrosine hydroxylase activity. Naunyn Schmiedebergs Arch Pharmacol. 1982 Aug;320(2):85–89. doi: 10.1007/BF00506305. [DOI] [PubMed] [Google Scholar]
- Buckland P. R., O'Donovan M. C., McGuffin P. Changes in dopa decarboxylase mRNA but not tyrosine hydroxylase mRNA levels in rat brain following antipsychotic treatment. Psychopharmacology (Berl) 1992;108(1-2):98–102. doi: 10.1007/BF02245292. [DOI] [PubMed] [Google Scholar]
- Buckland P. R., O'Donovan M. C., McGuffin P. Changes in dopamine D1, D2 and D3 receptor mRNA levels in rat brain following antipsychotic treatment. Psychopharmacology (Berl) 1992;106(4):479–483. doi: 10.1007/BF02244818. [DOI] [PubMed] [Google Scholar]
- Carlson J. H., Bergstrom D. A., Walters J. R. Stimulation of both D1 and D2 dopamine receptors appears necessary for full expression of postsynaptic effects of dopamine agonists: a neurophysiological study. Brain Res. 1987 Jan 6;400(2):205–218. doi: 10.1016/0006-8993(87)90619-6. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Christiansen J., Squires R. F. Antagonistic effects of apomorphine and haloperidol on rat striatal synaptosomal tyrosine hydroxylase. J Pharm Pharmacol. 1974 May;26(5):367–369. doi: 10.1111/j.2042-7158.1974.tb09293.x. [DOI] [PubMed] [Google Scholar]
- Clark D., White F. J. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1(4):347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- Cotman C. W. Isolation of synaptosomal and synaptic plasma membrane fractions. Methods Enzymol. 1974;31:445–452. doi: 10.1016/0076-6879(74)31050-6. [DOI] [PubMed] [Google Scholar]
- Deffond D., Durif F., Tournilhac M. Apomorphine in treatment of Parkinson's disease: comparison between subcutaneous and sublingual routes. J Neurol Neurosurg Psychiatry. 1993 Jan;56(1):101–103. doi: 10.1136/jnnp.56.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanoy R. L., Dunn A. J. Effects of haloperidol and apomorphine on catecholamine metabolism in brain slices. Reserpine-like effects of haloperidol. Biochem Pharmacol. 1982 Oct 15;31(20):3297–3305. doi: 10.1016/0006-2952(82)90564-0. [DOI] [PubMed] [Google Scholar]
- Frankel J. P., Lees A. J., Kempster P. A., Stern G. M. Subcutaneous apomorphine in the treatment of Parkinson's disease. J Neurol Neurosurg Psychiatry. 1990 Feb;53(2):96–101. doi: 10.1136/jnnp.53.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner C. R., Richards M. H. Use of D,L-alpha-monofluoromethyldopa to distinguish subcellular pools of aromatic amino acid decarboxylase in mouse brain. Brain Res. 1981 Jul 20;216(2):291–298. doi: 10.1016/0006-8993(81)90131-1. [DOI] [PubMed] [Google Scholar]
- Gershanik O., Heikkila R. E., Duvoisin R. C. Behavioral correlations of dopamine receptor activation. Neurology. 1983 Nov;33(11):1489–1492. doi: 10.1212/wnl.33.11.1489. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Freedman L. S., Backstrom T. The inhibition of catecholamine biosynthesis by apomorphine. J Pharm Pharmacol. 1970 Sep;22(9):715–717. doi: 10.1111/j.2042-7158.1970.tb12763.x. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M., Rossetti Z., Silvia C., Krajnc D., Neff N. H. Aromatic L-amino acid decarboxylase activity of the rat retina is modulated in vivo by environmental light. J Neurochem. 1988 Nov;51(5):1560–1564. doi: 10.1111/j.1471-4159.1988.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M., Wemlinger T. A., Sylvia C. P., Hubble J. P., Neff N. H. Aromatic L-amino acid decarboxylase activity of mouse striatum is modulated via dopamine receptors. J Neurochem. 1993 Jun;60(6):2175–2180. doi: 10.1111/j.1471-4159.1993.tb03503.x. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Joh T. H., Park D. H., Reis D. J. Direct phosphorylation of brain tyrosine hydroxylase by cyclic AMP-dependent protein kinase: mechanism of enzyme activation. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4744–4748. doi: 10.1073/pnas.75.10.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr W., Carlsson A., Lindqvist M., Magnusson T., Atack C. Evidence for a receptor-mediated feedback control of striatal tyrosine hydroxylase activity. J Pharm Pharmacol. 1972 Sep;24(9):744–747. doi: 10.1111/j.2042-7158.1972.tb09104.x. [DOI] [PubMed] [Google Scholar]
- Kurata K., Shibata R. Effects of D1 and D2 antagonists on the transient increase of dopamine release by dopamine agonists by means of brain dialysis. Neurosci Lett. 1991 Nov 25;133(1):77–80. doi: 10.1016/0304-3940(91)90061-w. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., WEISSBACH H., UDENFRIEND S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962 Jan;237:89–93. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieberman A., Goldstein M., Neophytides A., Kupersmith M., Leibowitz M., Zasorin N., Walker R., Kleinberg D. Lisuride in Parkinson disease: efficacy of lisuride compared to levodopa. Neurology. 1981 Aug;31(8):961–965. doi: 10.1212/wnl.31.8.961. [DOI] [PubMed] [Google Scholar]
- Magoski N. S., Walz W. Ionic dependence of a P2-purinoceptor mediated depolarization of cultured astrocytes. J Neurosci Res. 1992 Aug;32(4):530–538. doi: 10.1002/jnr.490320408. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Yamamoto T., Kato T. A new and highly sensitive voltammetric assay for aromatic L-amino acid decarboxylase activity by high-performance liquid chromatography. Anal Biochem. 1979 Nov 15;100(1):160–165. doi: 10.1016/0003-2697(79)90126-x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Okuno S., Fujisawa H. Accurate assay of dopa decarboxylase by preventing nonenzymatic decarboxylation of dopa. Anal Biochem. 1983 Mar;129(2):412–415. doi: 10.1016/0003-2697(83)90570-5. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Pilon C., Giros B., Sokoloff P., Martres M. P., Schwartz J. C. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991 Sep 12;353(6340):164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- Raese J. D., Edelman A. M., Makk G., Bruckwick E. A., Lovenberg W., Barchas J. D. Brain striatal tyrosine hydroxylase: activation of the enzyme by cyclic AMP-independent phosphorylation. Commun Psychopharmacol. 1979;3(5):295–301. [PubMed] [Google Scholar]
- Rosengarten H., Schweitzer J. W., Friedhoff A. J. Selective dopamine D2 receptor reduction enhances a D1-mediated oral dyskinesia in rats. Life Sci. 1986 Jul 7;39(1):29–35. doi: 10.1016/0024-3205(86)90434-0. [DOI] [PubMed] [Google Scholar]
- Rossetti Z. L., Silvia C. P., Krajnc D., Neff N. H., Hadjiconstantinou M. Aromatic L-amino acid decarboxylase is modulated by D1 dopamine receptors in rat retina. J Neurochem. 1990 Mar;54(3):787–791. doi: 10.1111/j.1471-4159.1990.tb02320.x. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M. Enzymatic isotopic assay for and presence of beta-phenylethylamine in brain. J Neurochem. 1974 Feb;22(2):211–216. doi: 10.1111/j.1471-4159.1974.tb11581.x. [DOI] [PubMed] [Google Scholar]
- Seeman P., Watanabe M., Grigoriadis D., Tedesco J. L., George S. R., Svensson U., Nilsson J. L., Neumeyer J. L. Dopamine D2 receptor binding sites for agonists. A tetrahedral model. Mol Pharmacol. 1985 Nov;28(5):391–399. [PubMed] [Google Scholar]
- Seiler M. P., Stoll A. P., Closse A., Frick W., Jaton A., Vigouret J. M. Structure-activity relationships of dopaminergic 5-hydroxy-2-aminotetralin derivatives with functionalized N-alkyl substituents. J Med Chem. 1986 Jun;29(6):912–917. doi: 10.1021/jm00156a007. [DOI] [PubMed] [Google Scholar]
- Sims K. L., Davis G. A., Bloom F. E. Activities of 3,4-dihydroxy-L-phenylalanine and 5-hydroxy-L-tryptophan decarboxylases in rat brain: assay characteristics and distribution. J Neurochem. 1973 Feb;20(2):449–464. doi: 10.1111/j.1471-4159.1973.tb12144.x. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981 Nov 26;294(5839):366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984 Dec 3;35(23):2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Vulliet P. R., Woodgett J. R., Cohen P. Phosphorylation of tyrosine hydroxylase by calmodulin-dependent multiprotein kinase. J Biol Chem. 1984 Nov 25;259(22):13680–13683. [PubMed] [Google Scholar]
- Walters J. R., Bergstrom D. A., Carlson J. H., Chase T. N., Braun A. R. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science. 1987 May 8;236(4802):719–722. doi: 10.1126/science.2953072. [DOI] [PubMed] [Google Scholar]
- Walters J. R., Roth R. H. Dopaminergic neurons: an in vivo system for measuring drug interactions with presynaptic receptors. Naunyn Schmiedebergs Arch Pharmacol. 1976 Dec;296(1):5–14. doi: 10.1007/BF00498834. [DOI] [PubMed] [Google Scholar]
- Weick B. G., Walters J. R. Effects of D1 and D2 dopamine receptor stimulation on the activity of substantia nigra pars reticulata neurons in 6-hydroxydopamine lesioned rats: D1/D2 coactivation induces potentiated responses. Brain Res. 1987 Mar 10;405(2):234–246. doi: 10.1016/0006-8993(87)90293-9. [DOI] [PubMed] [Google Scholar]
- Westerink B. H., Horn A. S. Do neuroleptics prevent the penetration of dopamine agonists into the brain? Eur J Pharmacol. 1979 Sep 1;58(1):39–48. doi: 10.1016/0014-2999(79)90337-6. [DOI] [PubMed] [Google Scholar]
- White F. J., Wang R. Y. Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J Neurosci. 1986 Jan;6(1):274–280. doi: 10.1523/JNEUROSCI.06-01-00274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. D., Russell M. P., Kurylo E., Newstead J. D. Stability of synaptosomal GABA levels and their use in determining the in vivo effects of drugs: convulsant agents. J Neurochem. 1979 Jul;33(1):61–68. doi: 10.1111/j.1471-4159.1979.tb11706.x. [DOI] [PubMed] [Google Scholar]
- Young E. A., Neff N. H., Hadjiconstantinou M. Evidence for cyclic AMP-mediated increase of aromatic L-amino acid decarboxylase activity in the striatum and midbrain. J Neurochem. 1993 Jun;60(6):2331–2333. doi: 10.1111/j.1471-4159.1993.tb03525.x. [DOI] [PubMed] [Google Scholar]
- Zhu M. Y., Juorio A. V., Paterson I. A., Boulton A. A. Regulation of aromatic L-amino acid decarboxylase by dopamine receptors in the rat brain. J Neurochem. 1992 Feb;58(2):636–641. doi: 10.1111/j.1471-4159.1992.tb09765.x. [DOI] [PubMed] [Google Scholar]
- Zhu M. Y., Juorio A. V., Paterson I. A., Boulton A. A. Regulation of striatal aromatic L-amino acid decarboxylase: effects of blockade or activation of dopamine receptors. Eur J Pharmacol. 1993 Jul 20;238(2-3):157–164. doi: 10.1016/0014-2999(93)90843-7. [DOI] [PubMed] [Google Scholar]


