Abstract
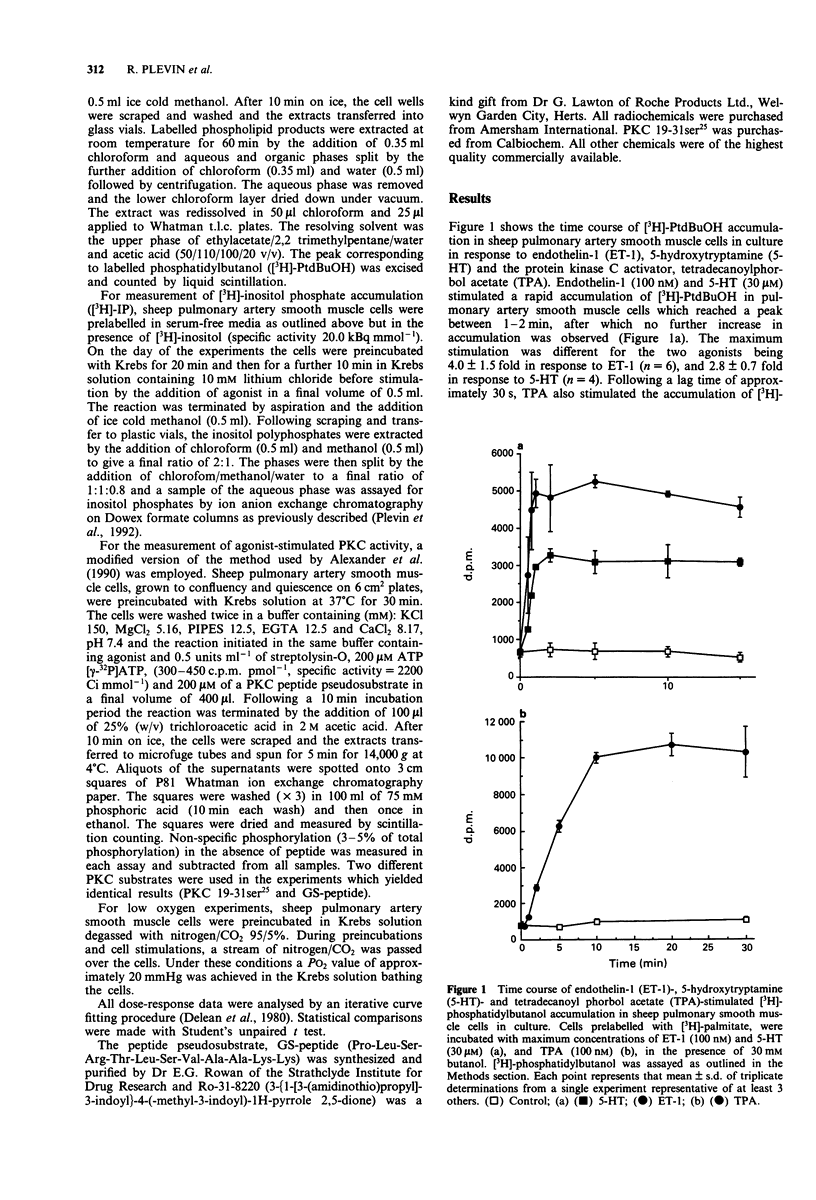
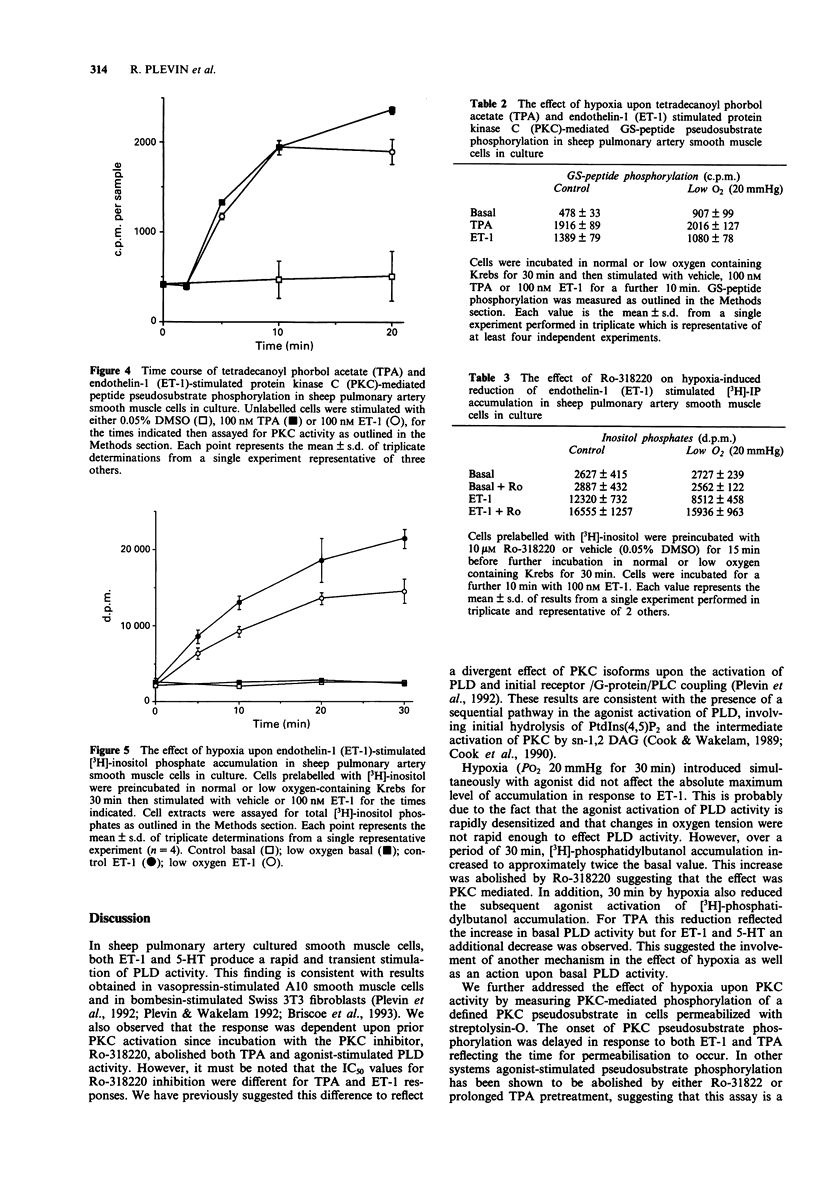
1. The aim of the study was to characterize the effects of hypoxia on agonist-stimulated phospholipase D (PLD) and phospholipase C activity of sheep pulmonary artery cultured smooth muscle cells. 2. Endothelin-1 (ET-1), 5-hydroxytryptamine (5-HT) and the protein kinase C (PKC) activator tetradecanoylphorbol acetate (TPA), stimulated a time- and concentration-dependent increase in [3H]-phosphatidylbutanol accumulation. This was abolished by pretreatment of the cells with the PKC inhibitor, Ro-318220, suggesting that agonist-stimulated phospholipase D activity is dependent upon the activation of PKC. 3. Hypoxia (PO2 20 mmHg for 30 min) stimulated basal [3H]-phosphatidylbutanol accumulation by approximately 2 fold and this activity was abolished by preincubation of the cells with 10 microM Ro-318220. 4. In cells preincubated in low O2 containing medium for 30 min, the subsequent agonist-stimulated accumulation of [3H]-phosphatidylbutanol was reduced. However, the decrease in stimulation was greater for ET-1 and 5-HT than for TPA. 5. ET-1 and TPA stimulated a time-dependent increase in protein kinase C- mediated psuedosubstrate phosphorylation. Following preincubation for 30 min in low O2 containing media, basal pseudosubstrate phosphorylation increased whilst the fold stimulation by TPA and ET-1 decreased. 6. In cells preincubated in low O2 containing medium, ET-1-stimulated [3H]-inositol phosphate accumulation was reduced by approximately 30-40%. This reduction was reversed by preincubation of the cells with Ro-318220. 7. These results suggest a role for PKC in the effects of hypoxia on PLD in pulmonary artery smooth muscle cells.
Full text
PDF
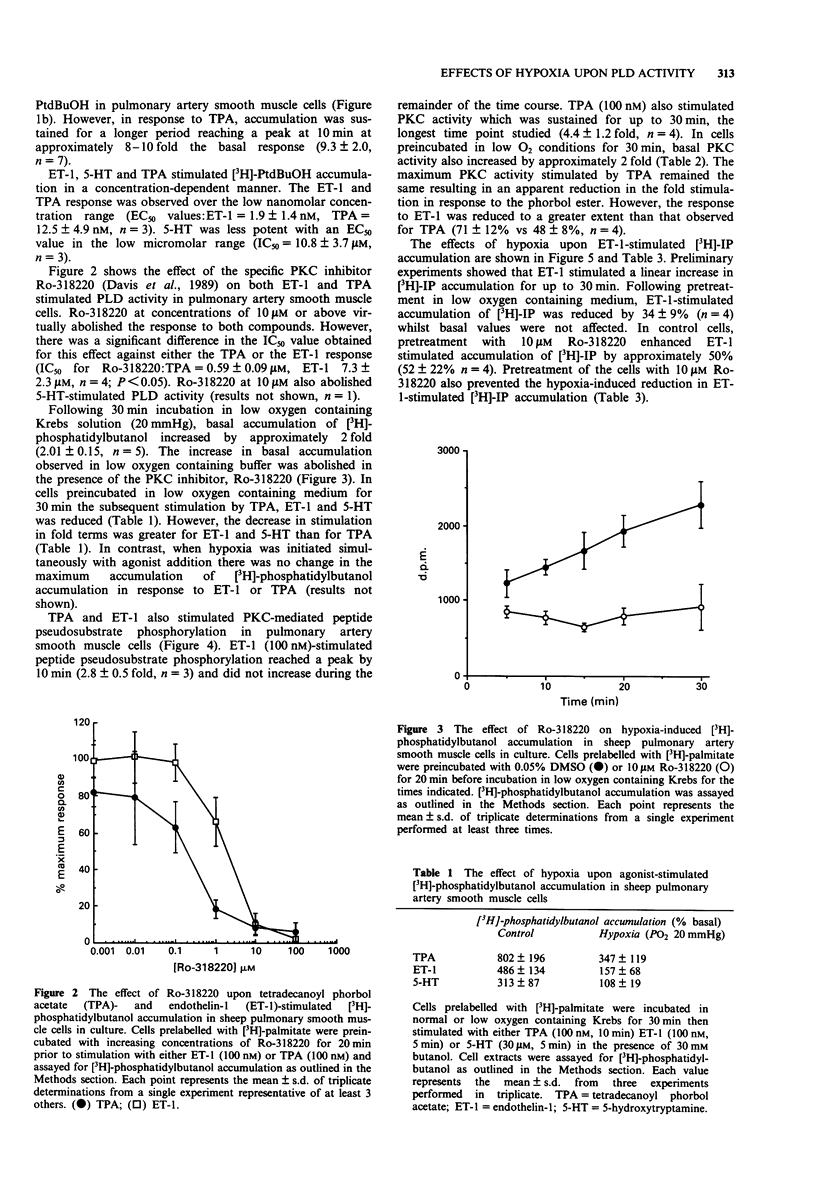

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Graves J. D., Lucas S. C., Cantrell D. A., Crumpton M. J. A method for measuring protein kinase C activity in permeabilized T lymphocytes by using peptide substrates. Evidence for multiple pathways of kinase activation. Biochem J. 1990 Jun 1;268(2):303–308. doi: 10.1042/bj2680303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarder M. R., Challiss R. A. Role of protein kinase C in the regulation of histamine and bradykinin stimulated inositol polyphosphate turnover in adrenal chromaffin cells. Br J Pharmacol. 1992 Dec;107(4):1140–1145. doi: 10.1111/j.1476-5381.1992.tb13420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A. J., Eagleton M. J., Wang D., Howell R. L., Strauch A. R., Khasgiwala V., Smith H. C. Induction of the proliferative phenotype in differentiated myogenic cells by hypoxia. J Biol Chem. 1991 Sep 25;266(27):18250–18258. [PubMed] [Google Scholar]
- Cook S. J., Palmer S., Plevin R., Wakelam M. J. Mass measurement of inositol 1,4,5-trisphosphate and sn-1,2-diacylglycerol in bombesin-stimulated Swiss 3T3 mouse fibroblasts. Biochem J. 1990 Jan 15;265(2):617–620. doi: 10.1042/bj2650617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dempsey E. C., McMurtry I. F., O'Brien R. F. Protein kinase C activation allows pulmonary artery smooth muscle cells to proliferate to hypoxia. Am J Physiol. 1991 Feb;260(2 Pt 1):L136–L145. doi: 10.1152/ajplung.1991.260.2.L136. [DOI] [PubMed] [Google Scholar]
- Lassègue B., Alexander R. W., Clark M., Griendling K. K. Angiotensin II-induced phosphatidylcholine hydrolysis in cultured vascular smooth-muscle cells. Regulation and localization. Biochem J. 1991 May 15;276(Pt 1):19–25. doi: 10.1042/bj2760019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNulty E. E., Plevin R., Wakelam M. J. Stimulation of the hydrolysis of phosphatidylinositol 4,5-bisphosphate and phosphatidylcholine by endothelin, a complete mitogen for Rat-1 fibroblasts. Biochem J. 1990 Dec 15;272(3):761–766. doi: 10.1042/bj2720761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray T. R., Chen L., Marshall B. E., Macarak E. J. Hypoxic contraction of cultured pulmonary vascular smooth muscle cells. Am J Respir Cell Mol Biol. 1990 Nov;3(5):457–465. doi: 10.1165/ajrcmb/3.5.457. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohanian J., Ollerenshaw J., Collins P., Heagerty A. Agonist-induced production of 1,2-diacylglycerol and phosphatidic acid in intact resistance arteries. Evidence that accumulation of diacylglycerol is not a prerequisite for contraction. J Biol Chem. 1990 May 25;265(15):8921–8928. [PubMed] [Google Scholar]
- Plevin R., Stewart A., Paul A., Wakelam M. J. Vasopressin-stimulated [3H]-inositol phosphate and [3H]-phosphatidylbutanol accumulation in A10 vascular smooth muscle cells. Br J Pharmacol. 1992 Sep;107(1):109–115. doi: 10.1111/j.1476-5381.1992.tb14471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakugi H., Tabuchi Y., Nakamaru M., Nagano M., Higashimori K., Mikami H., Ogihara T., Suzuki N. Evidence for endothelin-1 release from resistance vessels of rats in response to hypoxia. Biochem Biophys Res Commun. 1990 Jun 29;169(3):973–977. doi: 10.1016/0006-291x(90)91989-6. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Weber E., Bühler F. R. Phorbol ester promotes a sustained down-regulation of endothelin receptors and cellular responses to endothelin in human vascular smooth muscle cells. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1213–1219. doi: 10.1016/0006-291x(90)90995-y. [DOI] [PubMed] [Google Scholar]


