Abstract
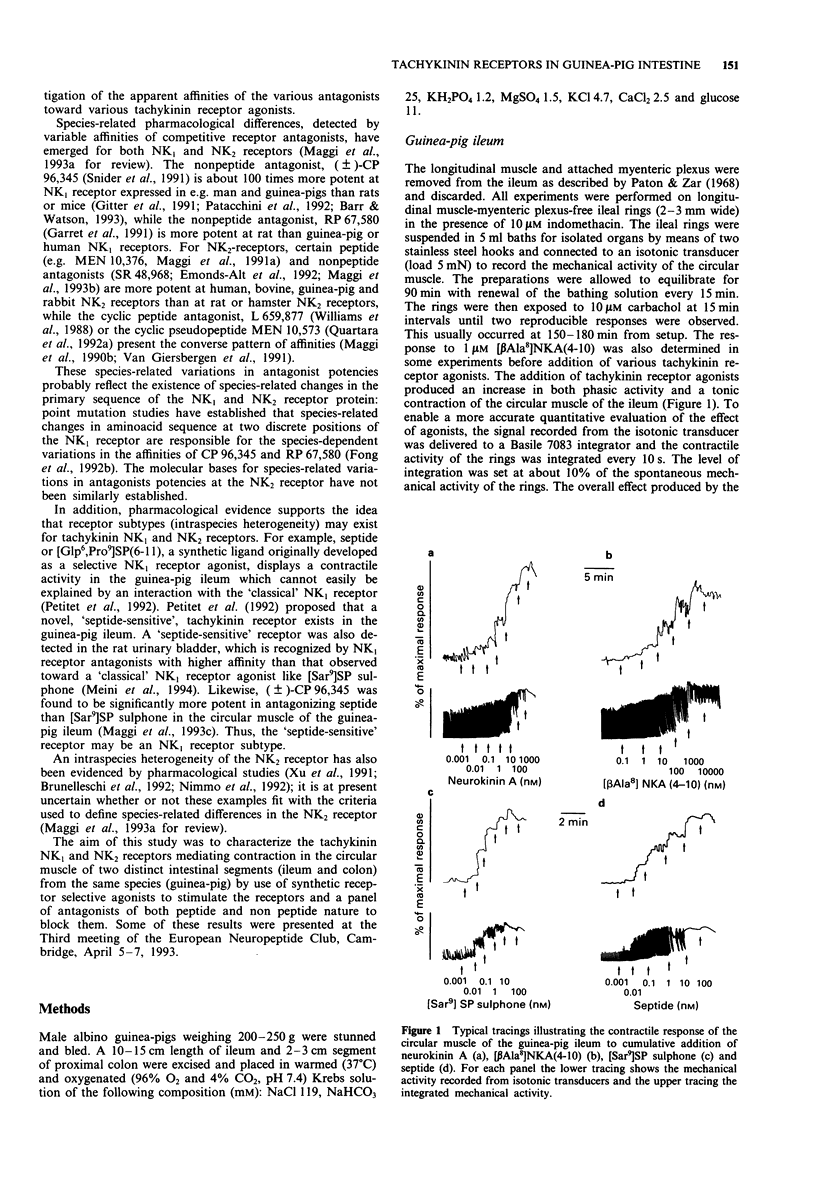
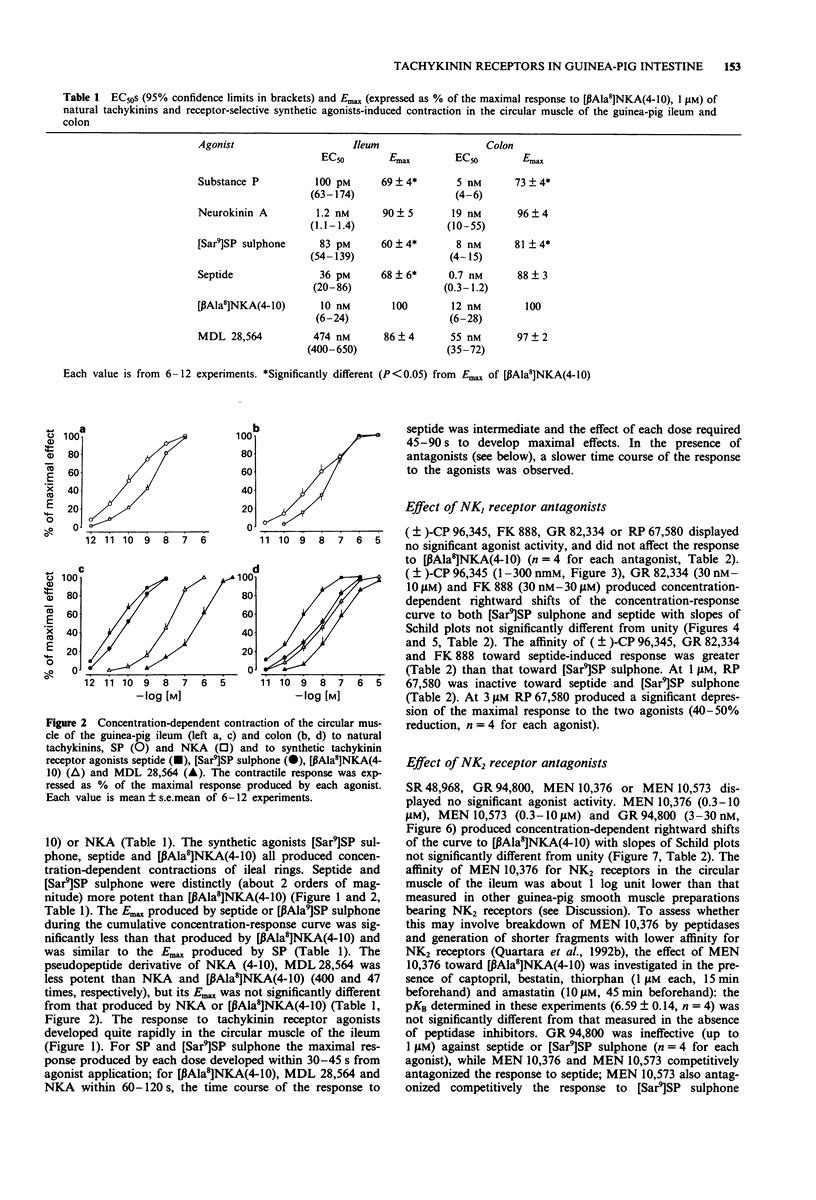
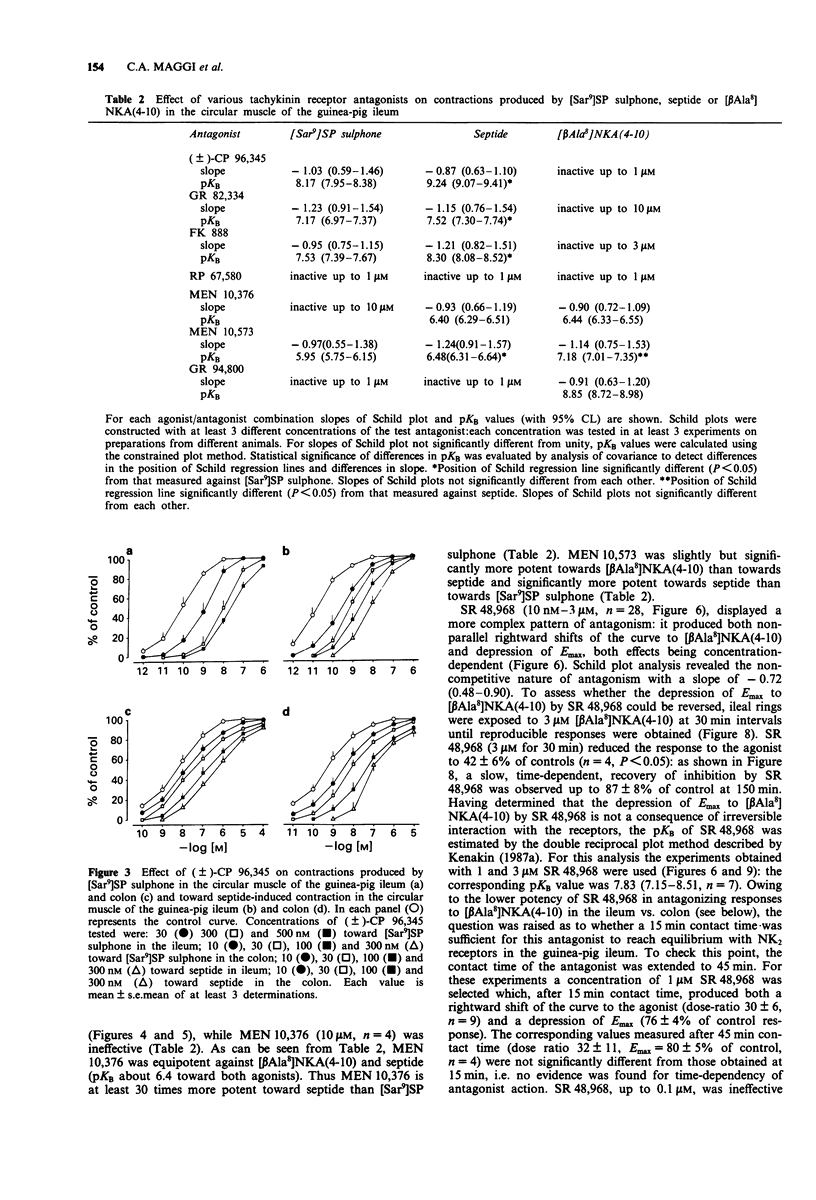
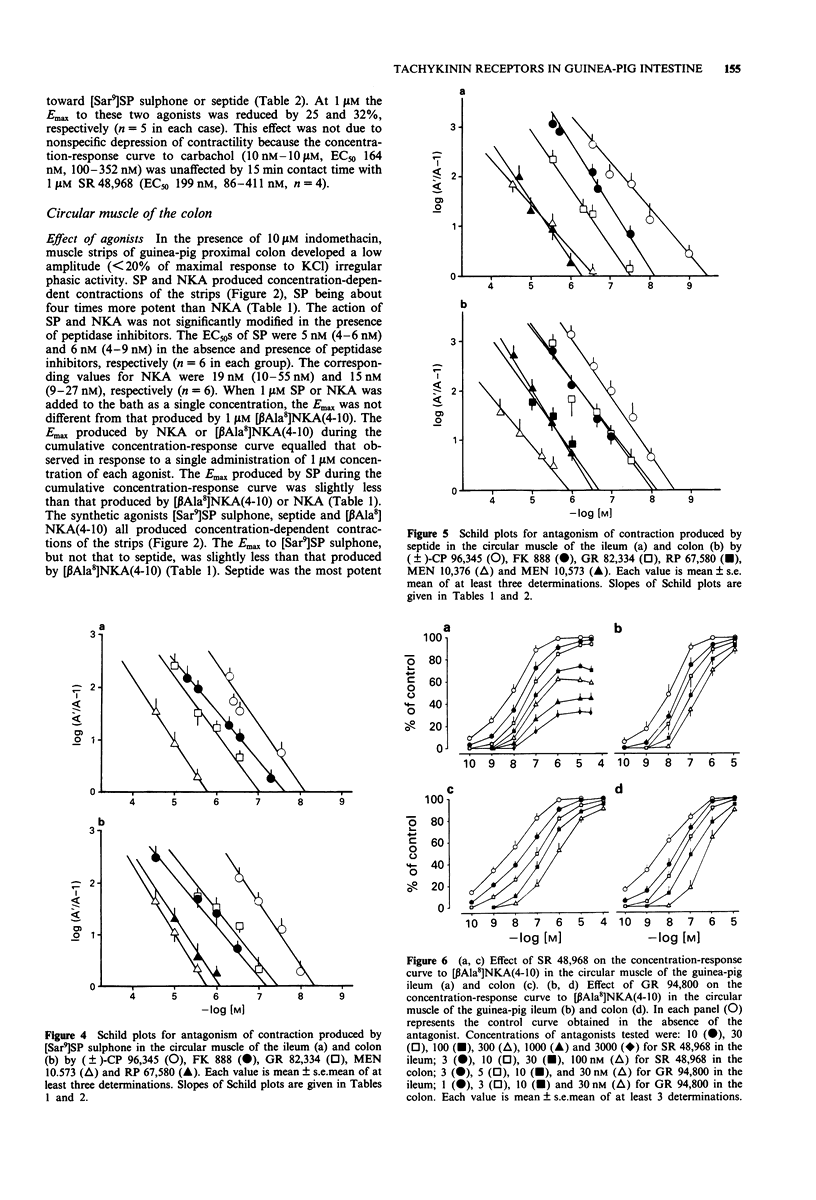
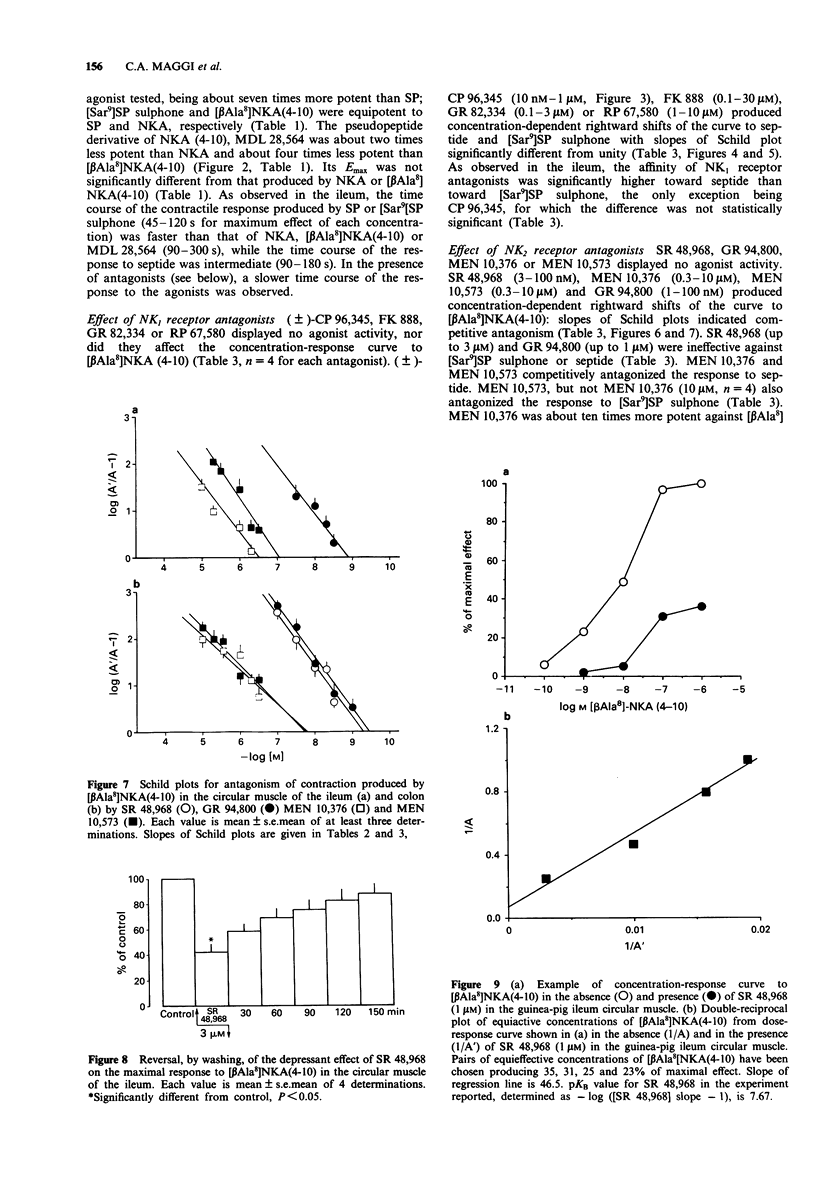
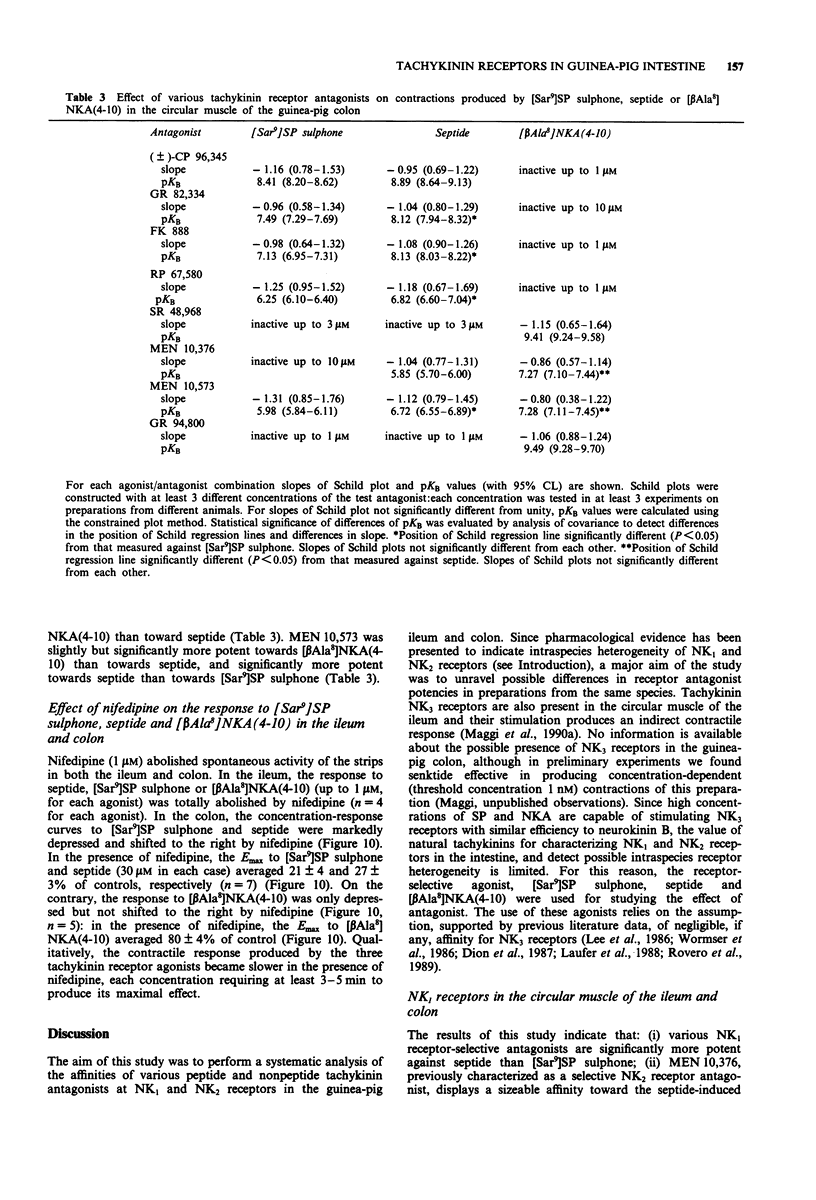
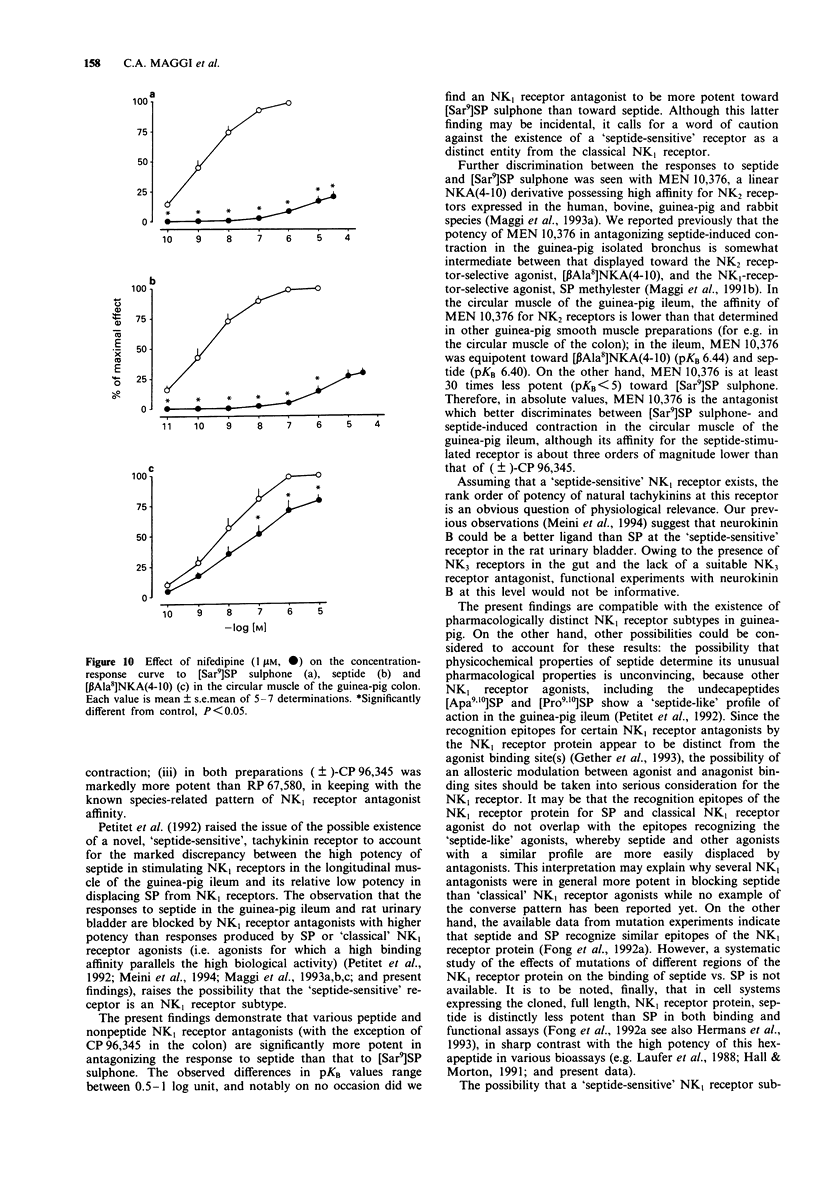
1. The aim of this study was the pharmacological characterization of tachykinin NK1 and NK2 receptors mediating contraction in the circular muscle of the guinea-pig ileum and proximal colon. The action of substance P (SP), neurokinin A (NKA) and of the synthetic agonists [Sar9]SP sulphone, [Glp6,Pro9]SP(6-11) (septide) and [beta Ala8]NKA(4-10) was investigated. The affinities of various peptide and nonpeptide antagonists for the NK1 and NK2 receptor was estimated by use of receptor selective agonists. 2. The natural agonists, SP and NKA, produced concentration-dependent contraction in both preparations. EC50 values were 100 pM and 5 nM for SP, 1.2 nM and 19 nM for NKA in the ileum and colon, respectively. The action of SP and NKA was not significantly modified by peptidase inhibitors (bestatin, captopril and thiorphan, 1 microM each). 3. Synthetic NK1 and NK2 receptor agonists produced concentration-dependent contraction of the circular muscle of the ileum and proximal colon. EC50 values were 83 pM, 36 pM and 10 nM in the ileum, 8 nM, 0.7 nM and 12 nM in the colon for [Sar9]SP sulphone, septide and [beta Ala8]NKA-(4-10), respectively. The pseudopeptide derivative of NKA(4-10), MDL 28,564 behaved as a full or near-to-full agonist in both preparations, its EC50s being 474 nM and 55 nM in the ileum and colon, respectively. 4. Nifedipine (1 microM) abolished the response to septide and [Sar9]SP sulphone in the ileum and produced a rightward shift and large depression of the response in the colon. The response to [beta Ala8]NKA(4-10) was abolished in the ileum and largely unaffected in the colon. 5. The NK1 receptor antagonists, (+/-)-CP 96,34, FK 888 and GR 82,334 competitively antagonized the response to septide and [Sar9]SP sulphone in both preparations without affecting that to [beta Ala8]NKA(4-10). In general, the NK1 receptor antagonists were significantly more potent toward septide than [Sar9]SP sulphone in both preparations. 6. The NK2 receptor antagonists, GR 94,800 and SR 48,968 selectively antagonized the response to [beta Ala8]NKA(4-10) without affecting that to [Sar9]SP sulphone or septide in the ileum and colon. SR 48,968 produced noncompetitive antagonism of the response to the NK2 receptor agonist in the ileum and competitive antagonism in the colon. 7. MEN 10,376 and the cyclic pseudopeptide MEN 10,573 antagonized in a competitive manner the response to [beta Ala8]NKA(4-10) in the ileum and colon.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr A. J., Watson S. P. Non-peptide antagonists, CP-96,345 and RP 67580, distinguish species variants in tachykinin NK1 receptors. Br J Pharmacol. 1993 Jan;108(1):223–227. doi: 10.1111/j.1476-5381.1993.tb13466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho L., Santicioli P., Patacchini R., Maggi C. A. Tachykininergic transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of NK2 receptors. Br J Pharmacol. 1992 Apr;105(4):805–810. doi: 10.1111/j.1476-5381.1992.tb09061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelleschi S., Ceni E., Fantozzi R., Maggi C. A. Evidence for tachykinin NK-2B-like receptors in guinea-pig alveolar macrophages. Life Sci. 1992;51(20):PL177–PL181. doi: 10.1016/0024-3205(92)90626-z. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Harbeson S. L., Hassmann C. F., 3rd, Shatzer S. A., Rouissi N., Nantel F., van Giersbergen P. L. [Leu9 psi(CH2NH)Leu10]-neurokinin A (4-10) (MDL 28,564) distinguishes tissue tachykinin peptide NK2 receptors. Life Sci. 1990;47(10):PL37–PL41. doi: 10.1016/0024-3205(90)90605-q. [DOI] [PubMed] [Google Scholar]
- Dion S., D'Orléans-Juste P., Drapeau G., Rhaleb N. E., Rouissi N., Tousignant C., Regoli D. Characterization of neurokinin receptors in various isolated organs by the use of selective agonists. Life Sci. 1987 Nov 16;41(20):2269–2278. doi: 10.1016/0024-3205(87)90538-8. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Anderson S. A., Yu H., Huang R. R., Strader C. D. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 1992 Jan;41(1):24–30. [PubMed] [Google Scholar]
- Fong T. M., Yu H., Huang R. R., Strader C. D. The extracellular domain of the neurokinin-1 receptor is required for high-affinity binding of peptides. Biochemistry. 1992 Dec 1;31(47):11806–11811. doi: 10.1021/bi00162a019. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Yu H., Strader C. D. Molecular basis for the species selectivity of the neurokinin-1 receptor antagonists CP-96,345 and RP67580. J Biol Chem. 1992 Dec 25;267(36):25668–25671. [PubMed] [Google Scholar]
- Fujii T., Murai M., Morimoto H., Maeda Y., Yamaoka M., Hagiwara D., Miyake H., Ikari N., Matsuo M. Pharmacological profile of a high affinity dipeptide NK1 receptor antagonist, FK888. Br J Pharmacol. 1992 Nov;107(3):785–789. doi: 10.1111/j.1476-5381.1992.tb14524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garret C., Carruette A., Fardin V., Moussaoui S., Peyronel J. F., Blanchard J. C., Laduron P. M. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard N. P., Bao L., Xiao-Ping H., Gerard C. Molecular aspects of the tachykinin receptors. Regul Pept. 1993 Jan 22;43(1-2):21–35. doi: 10.1016/0167-0115(93)90404-v. [DOI] [PubMed] [Google Scholar]
- Gether U., Yokota Y., Emonds-Alt X., Brelière J. C., Lowe J. A., 3rd, Snider R. M., Nakanishi S., Schwartz T. W. Two nonpeptide tachykinin antagonists act through epitopes on corresponding segments of the NK1 and NK2 receptors. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6194–6198. doi: 10.1073/pnas.90.13.6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitter B. D., Waters D. C., Bruns R. F., Mason N. R., Nixon J. A., Howbert J. J. Species differences in affinities of non-peptide antagonists for substance P receptors. Eur J Pharmacol. 1991 May 17;197(2-3):237–238. doi: 10.1016/0014-2999(91)90532-u. [DOI] [PubMed] [Google Scholar]
- Giuliani S., Lecci A., Giachetti A., Maggi C. A. Tachykinins and reflexly evoked atropine-resistant motility in the guinea pig colon in vivo. J Pharmacol Exp Ther. 1993 Jun;265(3):1224–1231. [PubMed] [Google Scholar]
- Hall J. M., Morton I. K. Novel selective agonists and antagonists confirm neurokinin NK1 receptors in guinea-pig vas deferens. Br J Pharmacol. 1991 Feb;102(2):511–517. doi: 10.1111/j.1476-5381.1991.tb12202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E., Jeanjean A. P., Fardin V., Pradier L., Garret C., Laduron P. M., Octave J. N., Maloteaux J. M. Interaction of the substance P receptor antagonist RP 67580 with the rat brain NK1 receptor expressed in transfected CHO cells. Eur J Pharmacol. 1993 Mar 15;245(1):43–50. doi: 10.1016/0922-4106(93)90167-8. [DOI] [PubMed] [Google Scholar]
- Kage R., Leeman S. E., Boyd N. D. Biochemical characterization of two different forms of the substance P receptor in rat submaxillary gland. J Neurochem. 1993 Jan;60(1):347–351. doi: 10.1111/j.1471-4159.1993.tb05857.x. [DOI] [PubMed] [Google Scholar]
- Laufer R., Gilon C., Chorev M., Selinger Z. Desensitization with a selective agonist discriminates between multiple tachykinin receptors. J Pharmacol Exp Ther. 1988 May;245(2):639–643. [PubMed] [Google Scholar]
- Lee C. M., Campbell N. J., Williams B. J., Iversen L. L. Multiple tachykinin binding sites in peripheral tissues and in brain. Eur J Pharmacol. 1986 Nov 4;130(3):209–217. doi: 10.1016/0014-2999(86)90270-0. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Ballati L., Lecci A., Manzini S., Patacchini R., Renzetti A. R., Rovero P., Quartara L., Giachetti A. In vivo evidence for tachykininergic transmission using a new NK-2 receptor-selective antagonist, MEN 10,376. J Pharmacol Exp Ther. 1991 Jun;257(3):1172–1178. [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Eglezos A., Quartara L., Giuliani S., Giachetti A. Tachykinin receptors in the guinea-pig renal pelvis: activation by exogenous and endogenous tachykinins. Br J Pharmacol. 1992 Sep;107(1):27–33. doi: 10.1111/j.1476-5381.1992.tb14459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Giachetti A., Meli A. Tachykinin receptors in the circular muscle of the guinea-pig ileum. Br J Pharmacol. 1990 Dec;101(4):996–1000. doi: 10.1111/j.1476-5381.1990.tb14195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Giuliani S., Giachetti A. In vivo and in vitro pharmacology of SR 48,968, a non-peptide tachykinin NK2 receptor antagonist. Eur J Pharmacol. 1993 Mar 30;234(1):83–90. doi: 10.1016/0014-2999(93)90709-q. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Meini S., Giuliani S. Evidence for the presence of a septide-sensitive tachykinin receptor in the circular muscle of the guinea-pig ileum. Eur J Pharmacol. 1993 Apr 28;235(2-3):309–311. doi: 10.1016/0014-2999(93)90152-8. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Quartara L., Rovero P., Santicioli P. Tachykinin receptors in the guinea-pig isolated bronchi. Eur J Pharmacol. 1991 May 17;197(2-3):167–174. doi: 10.1016/0014-2999(91)90517-t. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Rovero P., Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacol. 1993 Feb;13(1):23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- McElroy A. B., Clegg S. P., Deal M. J., Ewan G. B., Hagan R. M., Ireland S. J., Jordan C. C., Porter B., Ross B. C., Ward P. Highly potent and selective heptapeptide antagonists of the neurokinin NK-2 receptor. J Med Chem. 1992 Jul 10;35(14):2582–2591. doi: 10.1021/jm00092a008. [DOI] [PubMed] [Google Scholar]
- Patacchini R., Santicioli P., Astolfi M., Rovero P., Viti G., Maggi C. A. Activity of peptide and non-peptide antagonists at peripheral NK1 receptors. Eur J Pharmacol. 1992 Apr 29;215(1):93–98. doi: 10.1016/0014-2999(92)90613-9. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitet F., Saffroy M., Torrens Y., Lavielle S., Chassaing G., Loeuillet D., Glowinski J., Beaujouan J. C. Possible existence of a new tachykinin receptor subtype in the guinea pig ileum. Peptides. 1992 Mar-Apr;13(2):383–388. doi: 10.1016/0196-9781(92)90125-m. [DOI] [PubMed] [Google Scholar]
- Quartara L., Patacchini R., Giuliani S., Renzetti A. R., Rovero P., Maggi C. A. N-terminal truncated analogs of men 10376 as tachykinin NK-2 receptor antagonists. Life Sci. 1992;51(25):1929–1936. doi: 10.1016/0024-3205(92)90109-3. [DOI] [PubMed] [Google Scholar]
- Rovero P., Pestellini V., Rhaleb N. E., Dion S., Rouissi N., Tousignant C., Télémaque S., Drapeau G., Regoli D. Structure-activity studies of neurokinin A. Neuropeptides. 1989 May-Jun;13(4):263–270. doi: 10.1016/0143-4179(89)90080-2. [DOI] [PubMed] [Google Scholar]
- Snider R. M., Constantine J. W., Lowe J. A., 3rd, Longo K. P., Lebel W. S., Woody H. A., Drozda S. E., Desai M. C., Vinick F. J., Spencer R. W. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991 Jan 25;251(4992):435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- Wormser U., Laufer R., Hart Y., Chorev M., Gilon C., Selinger Z. Highly selective agonists for substance P receptor subtypes. EMBO J. 1986 Nov;5(11):2805–2808. doi: 10.1002/j.1460-2075.1986.tb04571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. J., Maggi C. A., Wiesenfeld-Hallin Z. On the role of NK-2 tachykinin receptors in the mediation of spinal reflex excitability in the rat. Neuroscience. 1991;44(2):483–490. doi: 10.1016/0306-4522(91)90071-u. [DOI] [PubMed] [Google Scholar]
- van Giersbergen P. L., Shatzer S. A., Henderson A. K., Lai J., Nakanishi S., Yamamura H. I., Buck S. H. Characterization of a tachykinin peptide NK2 receptor transfected into murine fibroblast B82 cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1661–1665. doi: 10.1073/pnas.88.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]


