Abstract
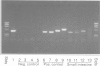
1 The polymerase chain reaction (PCR) was used in combination with plaque hybridization analysis to clone four variants of the EP3 prostaglandin receptor from a human small intestine cDNA library.
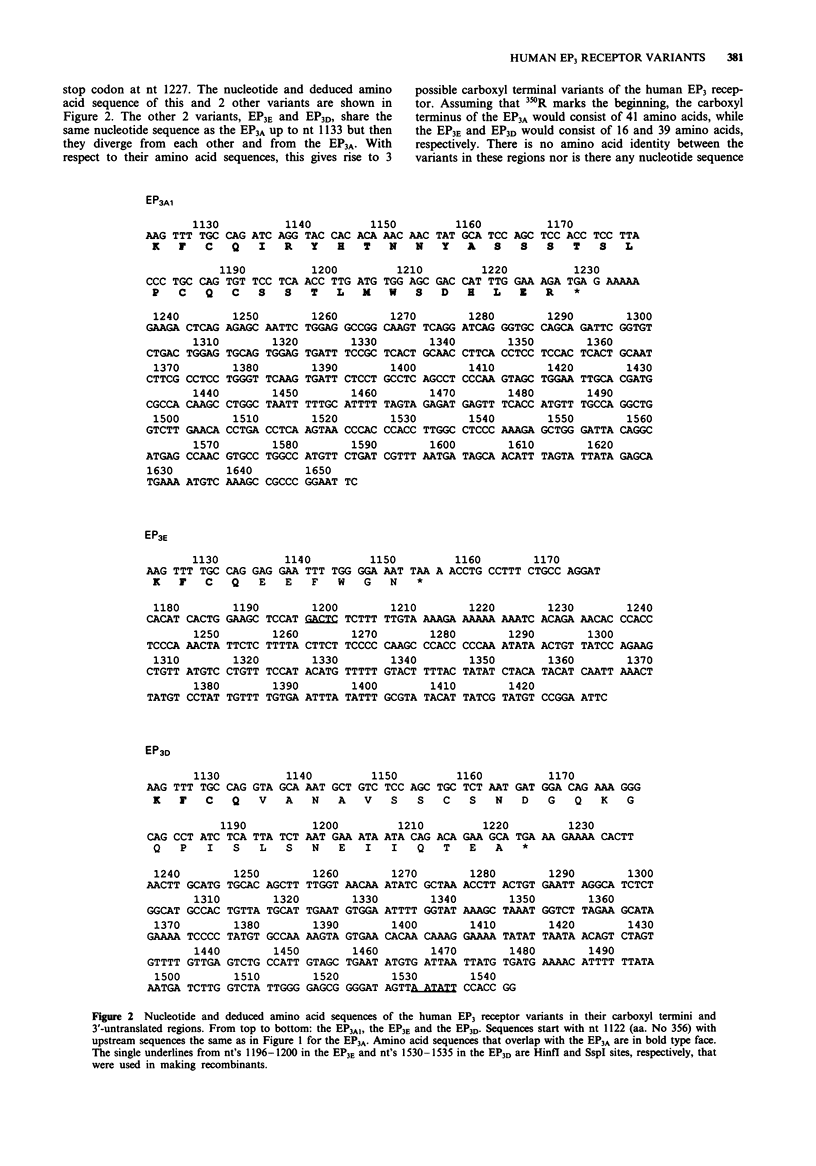
2 Three of these variants, i.e. the EP3A, EP3E and EP3D, share the same primary amino acid sequence except for their carboxyl termini, which diverge from one another at the same point, approximately 10 amino acids away from the end of the seventh membrane spanning domain of the receptor. The fourth variant (EP3A1) has a nucleotide coding sequence identical to EP3A but has a completely different 3′ untranslated sequence.
3 The carboxyl termini of the three isoforms differ most obviously in length with the EP3A being the longest (41 amino acids) and the EP3E being the shortest (16 amino acids). They also differ in content with the EP3A containing 9 serine and threonines in its carboxyl terminus and the EP3E none.
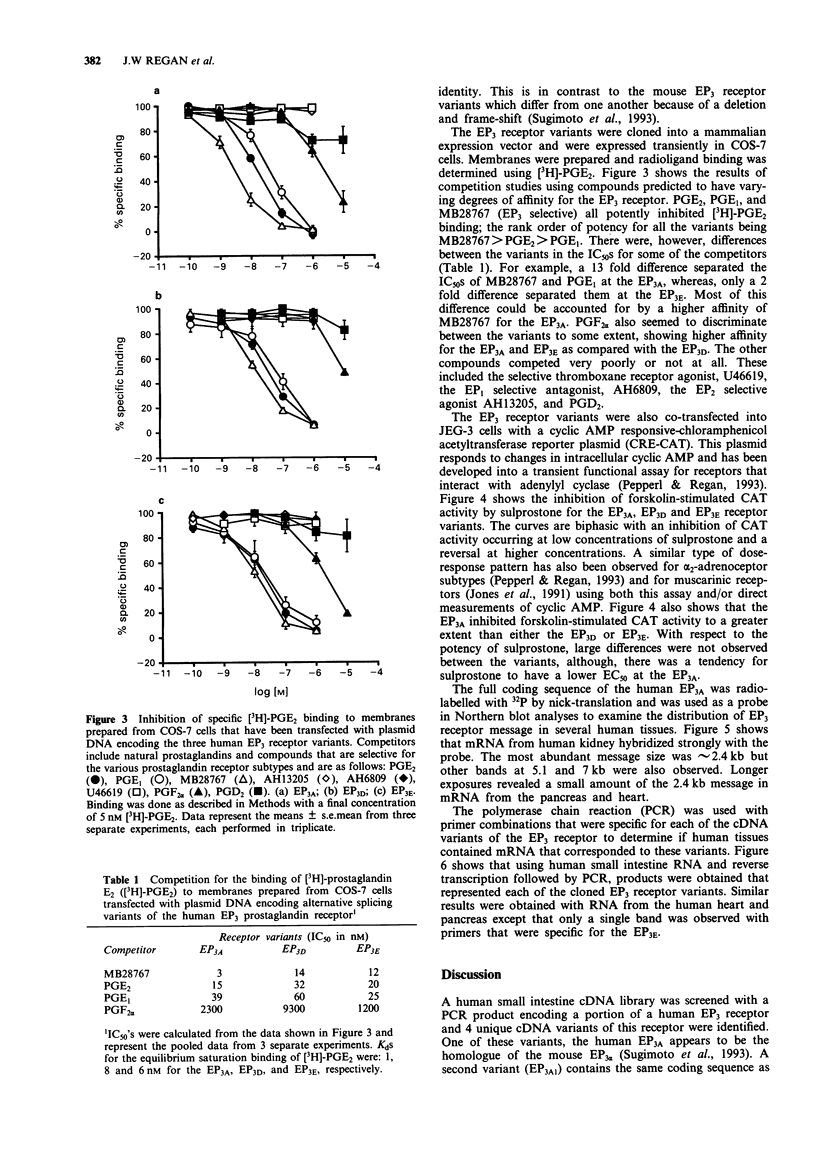
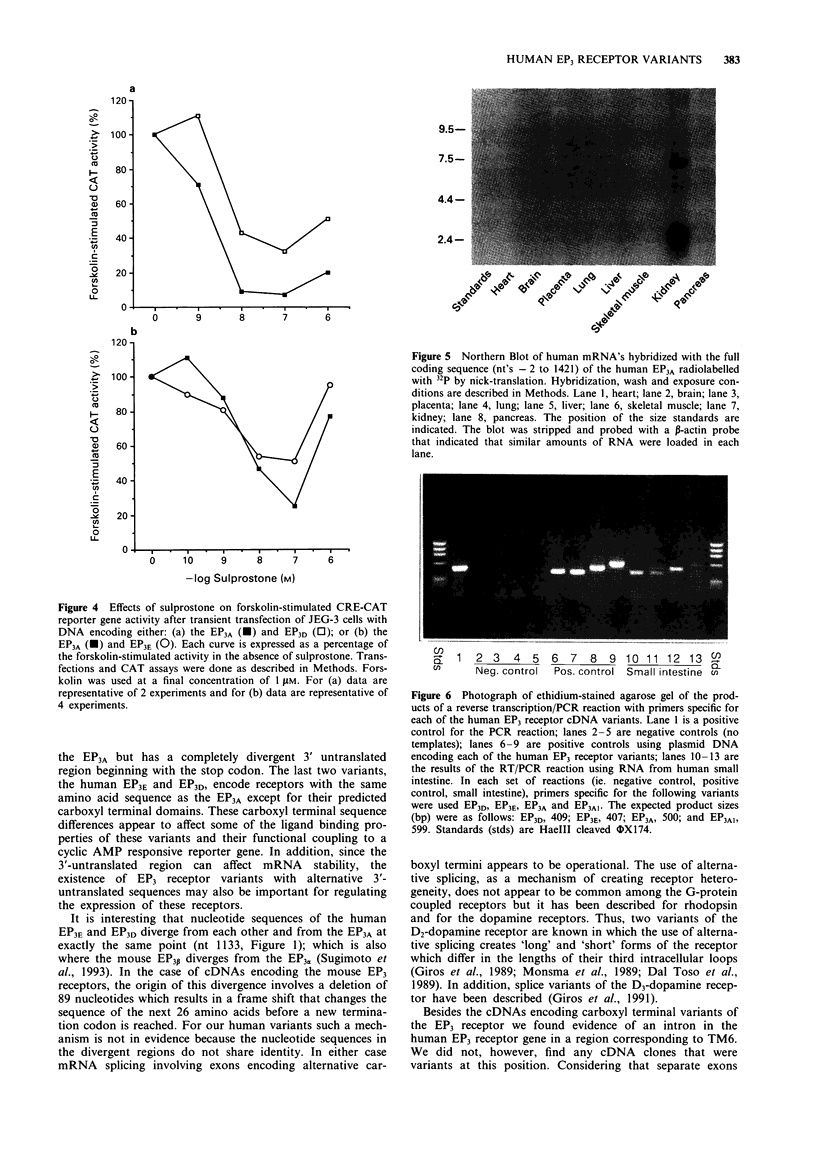
4 Transient expression in eukaryotic cells showed that the human EP3 receptor variants had similar but not identical radioligand binding properties and differed in their functional coupling to second messenger pathways. Up to 3 pmol mg-1 protein of [3H]-prostaglandin E2 binding could be obtained with more than 95% specific binding. Using a reporter gene assay, as a measure of intracellular cyclic AMP levels, the EP3A coupled more efficiently to the inhibition of adenylyl cyclase than did the EP3E.
5 PCR was used to confirm the presence of mRNAs encoding the four human EP3 receptor variants in tissues of the human small intestine, heart and pancreas. These findings indicate that the EP3 receptor variants identified here are likely to be expressed in tissues. The differences in the carboxyl termini at the protein level, and in the 3′ untranslated regions at the mRNA level, could be profound in terms of the regulation and functional coupling of these receptor isoforms.
Keywords: Prostaglandin, small intestine, cDNA, G-protein coupled, adenylyl cyclase, cyclic AMP inhibition, CAT assays
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furci L., Fitzgerald D. J., Fitzgerald G. A. Heterogeneity of prostaglandin H2/thromboxane A2 receptors: distinct subtypes mediate vascular smooth muscle contraction and platelet aggregation. J Pharmacol Exp Ther. 1991 Jul 1;258(1):74–81. [PubMed] [Google Scholar]
- Giles H., Leff P., Bolofo M. L., Kelly M. G., Robertson A. D. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol. 1989 Feb;96(2):291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Martres M. P., Pilon C., Sokoloff P., Schwartz J. C. Shorter variants of the D3 dopamine receptor produced through various patterns of alternative splicing. Biochem Biophys Res Commun. 1991 May 15;176(3):1584–1592. doi: 10.1016/0006-291x(91)90469-n. [DOI] [PubMed] [Google Scholar]
- Giros B., Sokoloff P., Martres M. P., Riou J. F., Emorine L. J., Schwartz J. C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989 Dec 21;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Hirata M., Hayashi Y., Ushikubi F., Yokota Y., Kageyama R., Nakanishi S., Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991 Feb 14;349(6310):617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Honda A., Sugimoto Y., Namba T., Watabe A., Irie A., Negishi M., Narumiya S., Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP2 subtype. J Biol Chem. 1993 Apr 15;268(11):7759–7762. [PubMed] [Google Scholar]
- Jones S. V., Heilman C. J., Brann M. R. Functional responses of cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1991 Aug;40(2):242–247. [PubMed] [Google Scholar]
- Lefkowitz R. J. G protein-coupled receptor kinases. Cell. 1993 Aug 13;74(3):409–412. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- Link R., Daunt D., Barsh G., Chruscinski A., Kobilka B. Cloning of two mouse genes encoding alpha 2-adrenergic receptor subtypes and identification of a single amino acid in the mouse alpha 2-C10 homolog responsible for an interspecies variation in antagonist binding. Mol Pharmacol. 1992 Jul;42(1):16–27. [PubMed] [Google Scholar]
- Masuda A., Mais D. E., Oatis J. E., Jr, Halushka P. V. Platelet and vascular thromboxane A2/prostaglandin H2 receptors. Evidence for different subclasses in the rat. Biochem Pharmacol. 1991 Jul 15;42(3):537–544. doi: 10.1016/0006-2952(91)90316-w. [DOI] [PubMed] [Google Scholar]
- Matthews J. S., Jones R. L. Potentiation of aggregation and inhibition of adenylate cyclase in human platelets by prostaglandin E analogues. Br J Pharmacol. 1993 Feb;108(2):363–369. doi: 10.1111/j.1476-5381.1993.tb12810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Namba T., Sugimoto Y., Negishi M., Irie A., Ushikubi F., Kakizuka A., Ito S., Ichikawa A., Narumiya S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993 Sep 9;365(6442):166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- Negishi M., Sugimoto Y., Irie A., Narumiya S., Ichikawa A. Two isoforms of prostaglandin E receptor EP3 subtype. Different COOH-terminal domains determine sensitivity to agonist-induced desensitization. J Biol Chem. 1993 May 5;268(13):9517–9521. [PubMed] [Google Scholar]
- Oksenberg D., Marsters S. A., O'Dowd B. F., Jin H., Havlik S., Peroutka S. J., Ashkenazi A. A single amino-acid difference confers major pharmacological variation between human and rodent 5-HT1B receptors. Nature. 1992 Nov 12;360(6400):161–163. doi: 10.1038/360161a0. [DOI] [PubMed] [Google Scholar]
- Pepperl D. J., Regan J. W. Selective coupling of alpha 2-adrenergic receptor subtypes to cyclic AMP-dependent reporter gene expression in transiently transfected JEG-3 cells. Mol Pharmacol. 1993 Oct;44(4):802–809. [PubMed] [Google Scholar]
- Regan J. W., Kobilka T. S., Yang-Feng T. L., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6301–6305. doi: 10.1073/pnas.85.17.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg W. K., Zhu J. H., Smith W. L. A prostaglandin E receptor coupled to a pertussis toxin-sensitive guanine nucleotide regulatory protein in rabbit cortical collecting tubule cells. J Biol Chem. 1990 May 25;265(15):8479–8483. [PubMed] [Google Scholar]
- Sugimoto Y., Namba T., Honda A., Hayashi Y., Negishi M., Ichikawa A., Narumiya S. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem. 1992 Apr 5;267(10):6463–6466. [PubMed] [Google Scholar]
- Sugimoto Y., Negishi M., Hayashi Y., Namba T., Honda A., Watabe A., Hirata M., Narumiya S., Ichikawa A. Two isoforms of the EP3 receptor with different carboxyl-terminal domains. Identical ligand binding properties and different coupling properties with Gi proteins. J Biol Chem. 1993 Feb 5;268(4):2712–2718. [PubMed] [Google Scholar]
- Takeuchi K., Abe T., Takahashi N., Abe K. Molecular cloning and intrarenal localization of rat prostaglandin E2 receptor EP3 subtype. Biochem Biophys Res Commun. 1993 Jul 30;194(2):885–891. doi: 10.1006/bbrc.1993.1904. [DOI] [PubMed] [Google Scholar]
- Valiquette M., Bonin H., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Involvement of tyrosine residues located in the carboxyl tail of the human beta 2-adrenergic receptor in agonist-induced down-regulation of the receptor. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5089–5093. doi: 10.1073/pnas.87.13.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Shimizu T., Nakao A., Taniguchi S., Arata Y., Teramoto T., Seyama Y., Ui M., Kurokawa K. Characterization of partially purified prostaglandin E2 receptor from the canine renal medulla: evidence for physical association of the receptor protein with the inhibitory guanine nucleotide-binding protein. Biochim Biophys Acta. 1991 Aug 6;1074(3):398–405. doi: 10.1016/0304-4165(91)90091-t. [DOI] [PubMed] [Google Scholar]
- Woodward D. F., Spada C. S., Hawley S. B., Williams L. S., Protzman C. E., Nieves A. L. Further studies on ocular responses to DP receptor stimulation. Eur J Pharmacol. 1993 Jan 19;230(3):327–333. doi: 10.1016/0014-2999(93)90569-4. [DOI] [PubMed] [Google Scholar]