Abstract
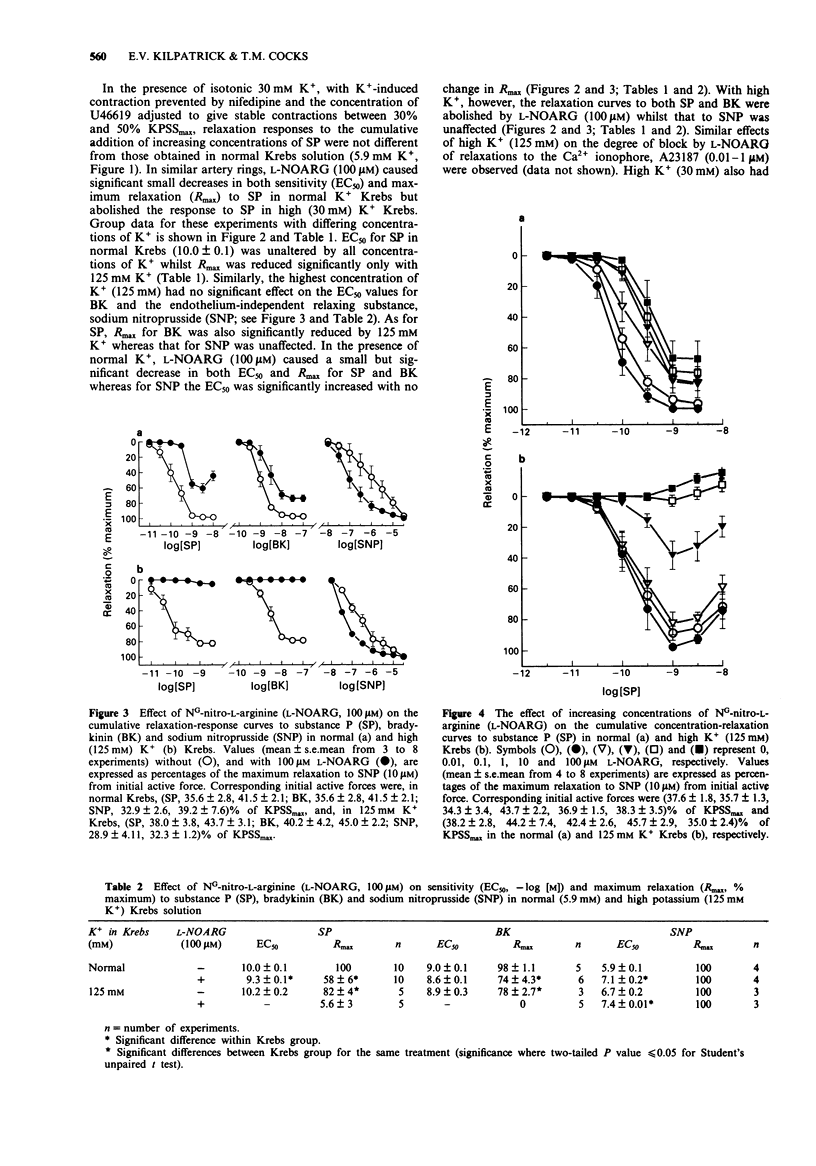
1. The possible roles of endothelial and smooth muscle cell hyperpolarization and nitric oxide (NO) in endothelium-dependent relaxation were examined in isolated rings of pig right coronary artery. 2. The effects of hyperpolarization were prevented with high K+ (30-125 mM), isotonic Krebs solutions. Functional antagonism due to high K(+)-induced smooth muscle contraction was prevented with 0.3 microM nifedipine (in all treatments, for consistency). All rings were contracted with the thromboxane-mimetic U46619, (1-100 nM) to bring them to an initial active force of within 30-50% of maximum contraction. 3. High K+ had no effects on the sensitivity (EC50) or time course of endothelium-dependent (substance P, SP; bradykinin, BK; calcimycin, A23187) and -independent (sodium nitroprusside, SNP) agents. Maximum relaxations (Rmax) to SP, BK and A23187 were reduced significantly by approximately 20% but only with 125 mM K+. 4. In normal K+ Krebs solution (5.9 mM), NG-nitro-L-arginine (L-NOARG; 100 microM) caused 40%, 20% and no reduction in Rmax for SP, BK and SNP respectively. EC50s for SP and BK were decreased significantly by approximately two fold whereas that for SNP was increased significantly by approximately ten fold. At all high K+ concentrations (30-125 mM), L-NOARG (100 microM) caused complete inhibition of relaxations to SP and BK but those to SNP were unaffected. 5. High K+ (30 mM) unmasked potent and concentration-dependent inhibition of relaxations of SP by L-NOARG. At 10 microM L-NOARG, all relaxation responses to SP were abolished and at the higher concentrations of SP (1-10 nM) small but significant contractions were observed.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeagbo A. S., Triggle C. R. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol. 1993 Mar;21(3):423–429. [PubMed] [Google Scholar]
- Angus J. A., Cocks T. M. Endothelium-derived relaxing factor. Pharmacol Ther. 1989;41(1-2):303–352. doi: 10.1016/0163-7258(89)90112-5. [DOI] [PubMed] [Google Scholar]
- Beny J. L., Brunet P. C., Huggel H. Effect of mechanical stimulation, substance P and vasoactive intestinal polypeptide on the electrical and mechanical activities of circular smooth muscles from pig coronary arteries contracted with acetylcholine: role of endothelium. Pharmacology. 1986;33(2):61–68. doi: 10.1159/000138202. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Moncada S., Pearson J. D., Mann G. E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br J Pharmacol. 1992 Apr;105(4):768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990 Sep;259(3 Pt 2):H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- Brunet P. C., Bény J. L. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26(4):228–234. doi: 10.1159/000158770. [DOI] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Bény J. L. Endothelial and smooth muscle cells hyperpolarized by bradykinin are not dye coupled. Am J Physiol. 1990 Mar;258(3 Pt 2):H836–H841. doi: 10.1152/ajpheart.1990.258.3.H836. [DOI] [PubMed] [Google Scholar]
- Bény J. L. Thimerosal hyperpolarizes arterial smooth muscles in an endothelium-dependent manner. Eur J Pharmacol. 1990 Aug 28;185(2-3):235–238. doi: 10.1016/0014-2999(90)90647-o. [DOI] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983 Oct 13;305(5935):627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- Cooke J. P., Rossitch E., Jr, Andon N. A., Loscalzo J., Dzau V. J. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991 Nov;88(5):1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C. L., Palacino J. J., Najibi S., Cohen R. A. Potassium channel-mediated relaxation to acetylcholine in rabbit arteries. J Pharmacol Exp Ther. 1993 Sep;266(3):1482–1489. [PubMed] [Google Scholar]
- Dwyer M. A., Bredt D. S., Snyder S. H. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991 May 15;176(3):1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- Elghozi J. L., Head G. A. Spinal noradrenergic pathways and pressor responses to central angiotensin II. Am J Physiol. 1990 Jan;258(1 Pt 2):H240–H246. doi: 10.1152/ajpheart.1990.258.1.H240. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Tominaga M., Ohmori S., Kobayashi K., Koga T., Takata Y., Fujishima M. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res. 1992 Apr;70(4):660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- Furfine E. S., Harmon M. F., Paith J. E., Garvey E. P. Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine. Biochemistry. 1993 Aug 24;32(33):8512–8517. doi: 10.1021/bi00084a017. [DOI] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschner K., Graier W. F., Kukovetz W. R. Activation of a small-conductance Ca(2+)-dependent K+ channel contributes to bradykinin-induced stimulation of nitric oxide synthesis in pig aortic endothelial cells. Biochim Biophys Acta. 1992 Oct 27;1137(2):162–170. doi: 10.1016/0167-4889(92)90198-k. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Busse R., Bassenge E. Endothelium-dependent hyperpolarization of smooth muscle cells in rabbit femoral arteries is not mediated by EDRF (nitric oxide). Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):438–442. doi: 10.1007/BF00172124. [DOI] [PubMed] [Google Scholar]
- Illiano S., Nagao T., Vanhoutte P. M. Calmidazolium, a calmodulin inhibitor, inhibits endothelium-dependent relaxations resistant to nitro-L-arginine in the canine coronary artery. Br J Pharmacol. 1992 Oct;107(2):387–392. doi: 10.1111/j.1476-5381.1992.tb12756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W. F., König A., Dambacher T., Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol. 1993 Jan;264(1 Pt 2):H238–H243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P. Voltage dependence of facilitated arginine flux mediated by the system y+ basic amino acid transporter. Biochemistry. 1993 Jun 8;32(22):5781–5785. doi: 10.1021/bi00073a009. [DOI] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Komori K., Vanhoutte P. M. Endothelium-derived hyperpolarizing factor. Blood Vessels. 1990;27(2-5):238–245. doi: 10.1159/000158815. [DOI] [PubMed] [Google Scholar]
- Lamontagne D., Pohl U., Busse R. Mechanical deformation of vessel wall and shear stress determine the basal release of endothelium-derived relaxing factor in the intact rabbit coronary vascular bed. Circ Res. 1992 Jan;70(1):123–130. doi: 10.1161/01.res.70.1.123. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993 Jun 15;268(17):12231–12234. [PubMed] [Google Scholar]
- Martin G. R., Bolofo M. L., Giles H. Inhibition of endothelium-dependent vasorelaxation by arginine analogues: a pharmacological analysis of agonist and tissue dependence. Br J Pharmacol. 1992 Mar;105(3):643–652. doi: 10.1111/j.1476-5381.1992.tb09033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkumyants A. M., Balashov S. A., Klimachev A. N., Kartamyshev S. P., Khayutin V. M. Nitric oxide does not mediate flow induced endothelium dependent arterial dilatation in the cat. Cardiovasc Res. 1992 Mar;26(3):256–260. doi: 10.1093/cvr/26.3.256. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S., Rees D. D., Schulz R., Palmer R. M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Characterization of endothelium-dependent relaxations resistant to nitro-L-arginine in the porcine coronary artery. Br J Pharmacol. 1992 Dec;107(4):1102–1107. doi: 10.1111/j.1476-5381.1992.tb13414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J Physiol. 1992 Jan;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Olesen S. P., Davies P. F., Clapham D. E. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988 Jun;62(6):1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Olken N. M., Rusche K. M., Richards M. K., Marletta M. A. Inactivation of macrophage nitric oxide synthase activity by NG-methyl-L-arginine. Biochem Biophys Res Commun. 1991 Jun 14;177(2):828–833. doi: 10.1016/0006-291x(91)91864-9. [DOI] [PubMed] [Google Scholar]
- Pacicca C., von der Weid P. Y., Beny J. L. Effect of nitro-L-arginine on endothelium-dependent hyperpolarizations and relaxations of pig coronary arteries. J Physiol. 1992 Nov;457:247–256. doi: 10.1113/jphysiol.1992.sp019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard V., Tanner F. C., Tschudi M., Lüscher T. F. Different activation of L-arginine pathway by bradykinin, serotonin, and clonidine in coronary arteries. Am J Physiol. 1990 Nov;259(5 Pt 2):H1433–H1439. doi: 10.1152/ajpheart.1990.259.5.H1433. [DOI] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeffter P., Miller R. C. Role of sodium-calcium exchange and effects of calcium entry blockers on endothelial-mediated responses in rat isolated aorta. Mol Pharmacol. 1986 Jul;30(1):53–57. [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Xiao X. H., Mussap C. J., Burcher E. Characterization of the tachykinin NK2 receptor subtype in the rabbit pulmonary artery. Peptides. 1992 Mar-Apr;13(2):281–285. doi: 10.1016/0196-9781(92)90109-g. [DOI] [PubMed] [Google Scholar]
- van den Brink F. G. The model of functional interaction. I. Development and first check of a new model of functional synergism and antagonism. Eur J Pharmacol. 1973 Jun;22(3):270–278. doi: 10.1016/0014-2999(73)90026-5. [DOI] [PubMed] [Google Scholar]
- van den Brink F. G. The model of functional interaction. II. Experimental verification of a new model: the antagonism of beta-adrenoceptor stimulants and other agonists. Eur J Pharmacol. 1973 Jun;22(3):279–286. doi: 10.1016/0014-2999(73)90027-7. [DOI] [PubMed] [Google Scholar]
- von der Weid P. Y., Bény J. L. Effect of Ca2+ ionophores on membrane potential of pig coronary artery endothelial cells. Am J Physiol. 1992 Jun;262(6 Pt 2):H1823–H1831. doi: 10.1152/ajpheart.1992.262.6.H1823. [DOI] [PubMed] [Google Scholar]


