Abstract
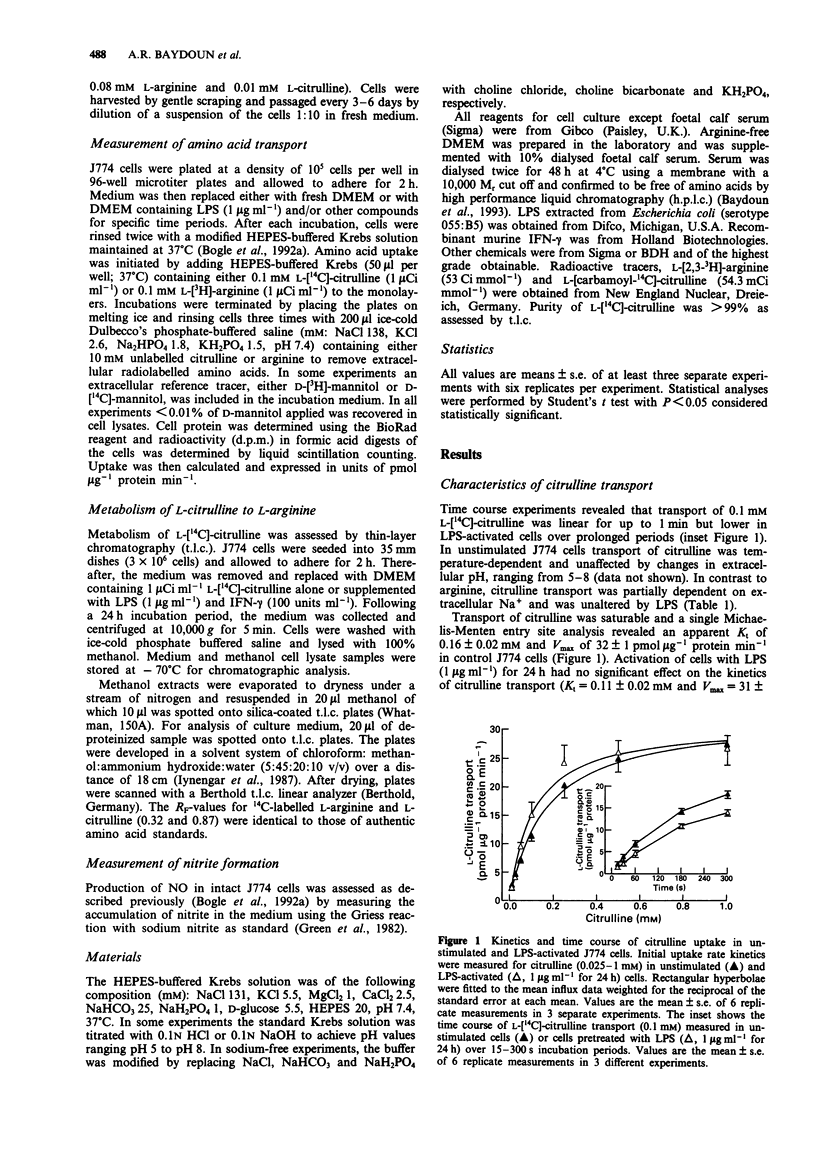
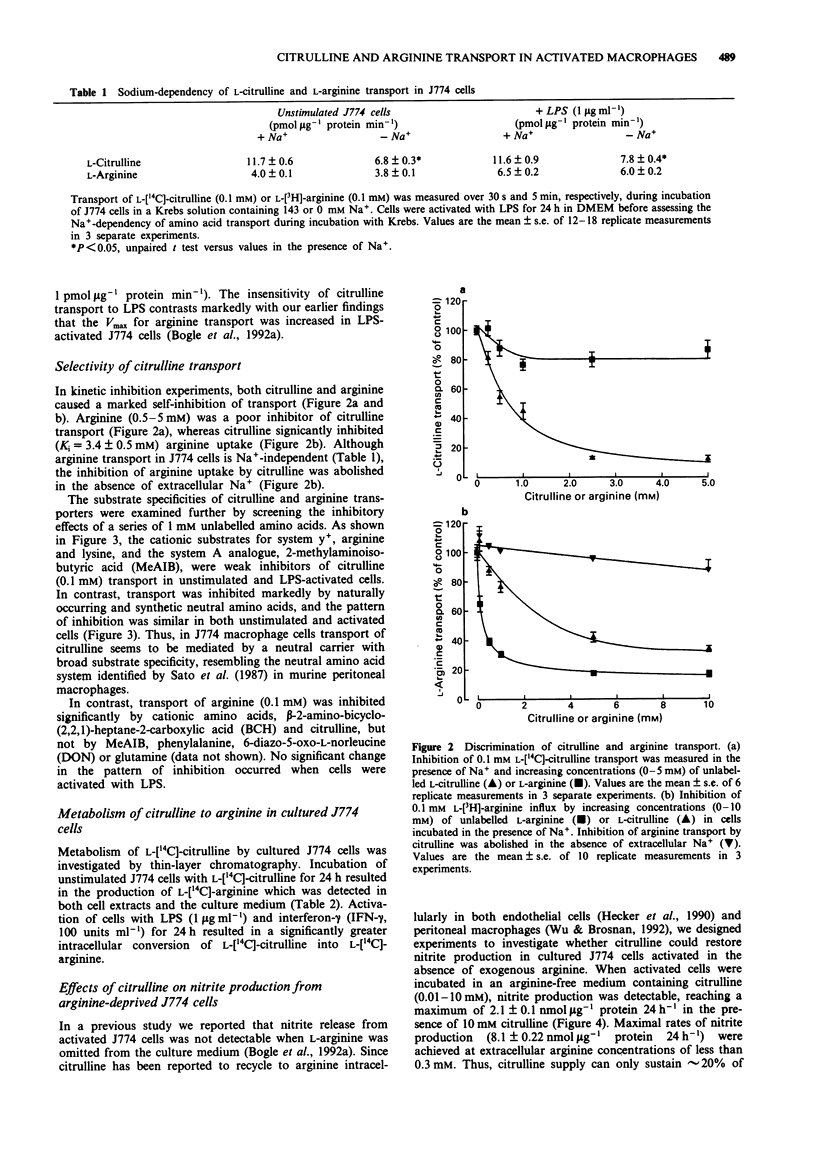
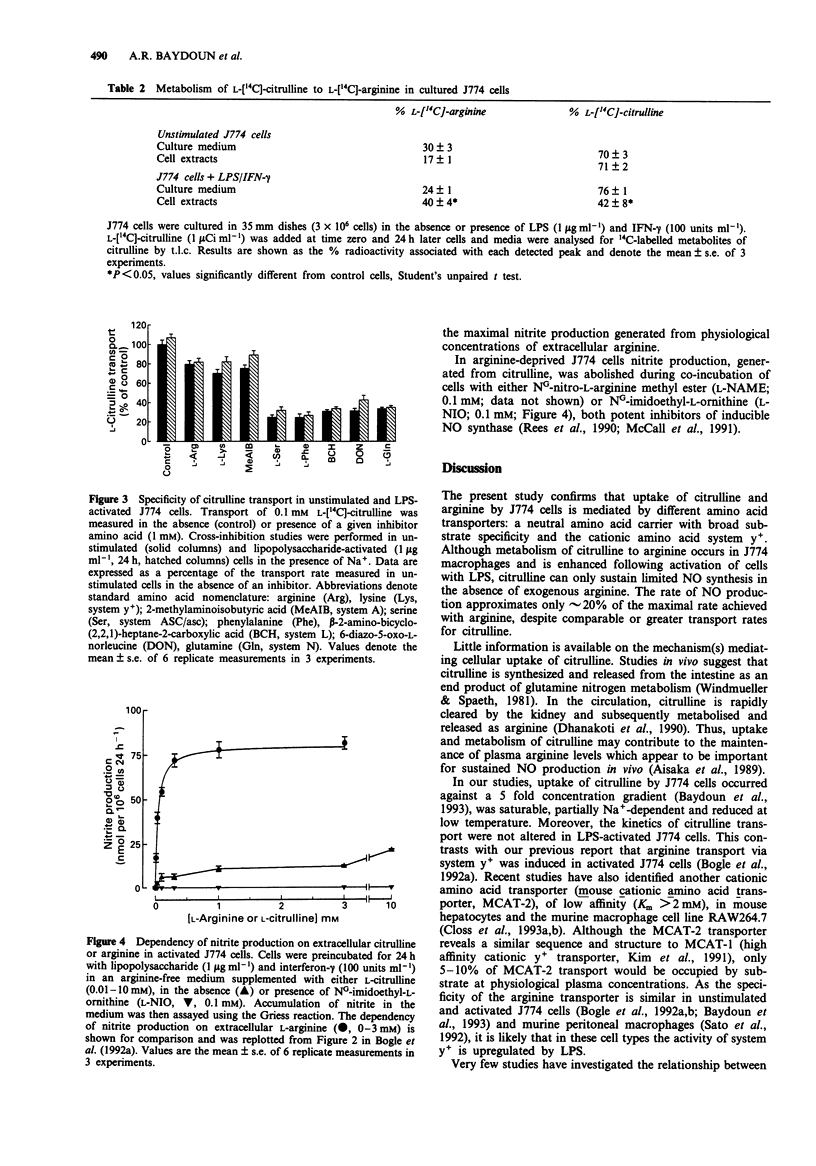
1. The kinetics, specificity, pH- and Na(+)-dependency of L-citrulline transport were examined in unstimulated and lipopolysaccharide (LPS)-activated murine macrophage J774 cells. The dependency of nitric oxide production on extracellular arginine or citrulline was investigated in cells activated with LPS (1 microgram ml-1) for 24 h. 2. In unstimulated J774 cells, transport of citrulline was saturable (Kt = 0.16 mM and Vmax = 32 pmol micrograms-1 protein min-1), pH-insensitive and partially Na(+)-dependent. In contrast to arginine, transport of citrulline was unchanged in LPS-activated (1 microgram ml-1, 24 h) cells. 3. Kinetic inhibition experiments revealed that arginine was a relatively poor inhibitor of citrulline transport, whilst citrulline was a more potent inhibitor (Ki = 3.4 mM) of arginine transport but only in the presence of extracellular Na+. Neutral amino acids inhibited citrulline transport (Ki = 0.2-0.3 mM), but were poor inhibitors of arginine transport. 4. Activated J774 cells did not release nitrite in the absence of exogenous arginine. Addition of citrulline (0.01-10 mM), in the absence of exogenous arginine, could only partially restore the ability of cells to synthesize nitrite, which was abolished by 100 microM NG-nitro-L-arginine methyl ester or NG-iminoethyl-L-ornithine. 5. Intracellular metabolism of L-[14C]-citrulline to L-[14C]-arginine was detected in unstimulated J774 cells and was increased further in cells activated with LPS and interferon-gamma. 6. We conclude that J774 macrophage cells transport citrulline via a saturable but nonselective neutral carrier which is insensitive to induction by LPS.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- Albina J. E., Mills C. D., Henry W. L., Jr, Caldwell M. D. Regulation of macrophage physiology by L-arginine: role of the oxidative L-arginine deiminase pathway. J Immunol. 1989 Dec 1;143(11):3641–3646. [PubMed] [Google Scholar]
- Assreuy J., Moncada S. A perfusion system for the long term study of macrophage activation. Br J Pharmacol. 1992 Oct;107(2):317–321. doi: 10.1111/j.1476-5381.1992.tb12744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun A. R., Bogle R. G., Pearson J. D., Mann G. E. Selective inhibition by dexamethasone of induction of NO synthase, but not of induction of L-arginine transport, in activated murine macrophage J774 cells. Br J Pharmacol. 1993 Dec;110(4):1401–1406. doi: 10.1111/j.1476-5381.1993.tb13976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogle R. G., Baydoun A. R., Pearson J. D., Moncada S., Mann G. E. L-arginine transport is increased in macrophages generating nitric oxide. Biochem J. 1992 May 15;284(Pt 1):15–18. doi: 10.1042/bj2840015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N., Antonioli J. A. Cationic amino acid transport in the rabbit reticulocyte. Na+-dependent inhibition of Na+-independent transport. J Biol Chem. 1969 Mar 25;244(6):1497–1504. [PubMed] [Google Scholar]
- Closs E. I., Albritton L. M., Kim J. W., Cunningham J. M. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J Biol Chem. 1993 Apr 5;268(10):7538–7544. [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Dhanakoti S. N., Brosnan J. T., Herzberg G. R., Brosnan M. E. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol. 1990 Sep;259(3 Pt 1):E437–E442. doi: 10.1152/ajpendo.1990.259.3.E437. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg G. R., Sheerin H., Lerner J. Cationic amino acid transport in chicken small intestine. Comp Biochem Physiol A Comp Physiol. 1971 Sep 1;40(1):229–247. doi: 10.1016/0300-9629(71)90163-0. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Kreusch J., Maier K. P., Munder P. G., Decker K. The urea cycle in different types of macrophages. Biochem Soc Trans. 1978;6(5):990–993. doi: 10.1042/bst0060990. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Geiges M., Keist R. L-arginine-dependent reactive nitrogen intermediates as mediators of tumor cell killing by activated macrophages. Cancer Res. 1990 Mar 1;50(5):1421–1425. [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Lawless K., Maenz D., Cheeseman C. Is leucine an allosteric modulator of the lysine transporter in the intestinal basolateral membrane? Am J Physiol. 1987 Nov;253(5 Pt 1):G637–G642. doi: 10.1152/ajpgi.1987.253.5.G637. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Feelisch M., Palmer R. M., Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991 Jan;102(1):234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Munck B. G. Lysine transport across the small intestine. Stimulating and inhibitory effects of neutral amino acids. J Membr Biol. 1980 Mar 31;53(1):45–53. doi: 10.1007/BF01871171. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Fujiwara M., Bannai S. Effect of lipopolysaccharide on transport and metabolism of arginine in mouse peritoneal macrophages. J Leukoc Biol. 1992 Aug;52(2):161–164. doi: 10.1002/jlb.52.2.161. [DOI] [PubMed] [Google Scholar]
- Sato H., Ishii T., Sugita Y., Bannai S. Induction of cationic amino acid transport activity in mouse peritoneal macrophages by lipopolysaccharide. Biochim Biophys Acta. 1991 Oct 14;1069(1):46–52. doi: 10.1016/0005-2736(91)90102-e. [DOI] [PubMed] [Google Scholar]
- Sato H., Watanabe H., Ishii T., Bannai S. Neutral amino acid transport in mouse peritoneal macrophages. J Biol Chem. 1987 Sep 25;262(27):13015–13019. [PubMed] [Google Scholar]
- Sweiry J. H., Muñoz M., Mann G. E. Cis-inhibition and trans-stimulation of cationic amino acid transport in the perfused rat pancreas. Am J Physiol. 1991 Sep;261(3 Pt 1):C506–C514. doi: 10.1152/ajpcell.1991.261.3.C506. [DOI] [PubMed] [Google Scholar]
- Takema M., Inaba K., Uno K., Kakihara K., Tawara K., Muramatsu S. Effect of L-arginine on the retention of macrophage tumoricidal activity. J Immunol. 1991 Mar 15;146(6):1928–1933. [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Source and fate of circulating citrulline. Am J Physiol. 1981 Dec;241(6):E473–E480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- Wu G. Y., Brosnan J. T. Macrophages can convert citrulline into arginine. Biochem J. 1992 Jan 1;281(Pt 1):45–48. doi: 10.1042/bj2810045. [DOI] [PMC free article] [PubMed] [Google Scholar]


