Abstract
1. The present study examined the responses of the rabbit carotid artery to five vasoconstrictors after neo-intima formation induced by perivascular collar treatment and evaluated the role of constitutive and inducible nitric oxide (NO) synthase and endothelial cells (ECs). 2. Ring segments of the rabbit carotid artery were mounted in organ chambers for isometric tension recording. Neo-intima-bearing vessels developed less force (Emax) in response to KCl, the thromboxane-mimetic U-46619 and 5-hydroxytryptamine (5-HT), but not to angiotensin I and II. 3. The collar-treatment increased the sensitivity to 5-HT, and decreased the sensitivity to angiotensin II. The sensitivity to U-46619 and angiotensin I remained unchanged. 4. Mechanical removal of ECs and inhibition of NO biosynthesis by NG-monomethyl-L-arginine (L-NMMA) and NG-nitro-L-arginine (L-NOARG) increased the sensitivity to 5-HT in sham and collar-treated segments to the same extent. The effects of collar-treatment and endothelial removal or treatment with inhibitors of NO biosynthesis were additive. Inhibition of NO biosynthesis failed to augment sensitivity to 5-HT after endothelial denudation. L-NOARG increased the force development to KCl in sham and collar-treated segments to the same extent. However, L-NMMA and L-NOARG failed to augment the contractile responses of neo-intima-bearing vessels to 5-HT and KCl after endothelial removal. 5. The responses to angiotensin I were not altered, either by the neo-intima or by endothelial removal. In arteries with a neo-intima the sensitivity to angiotensin II was decreased.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

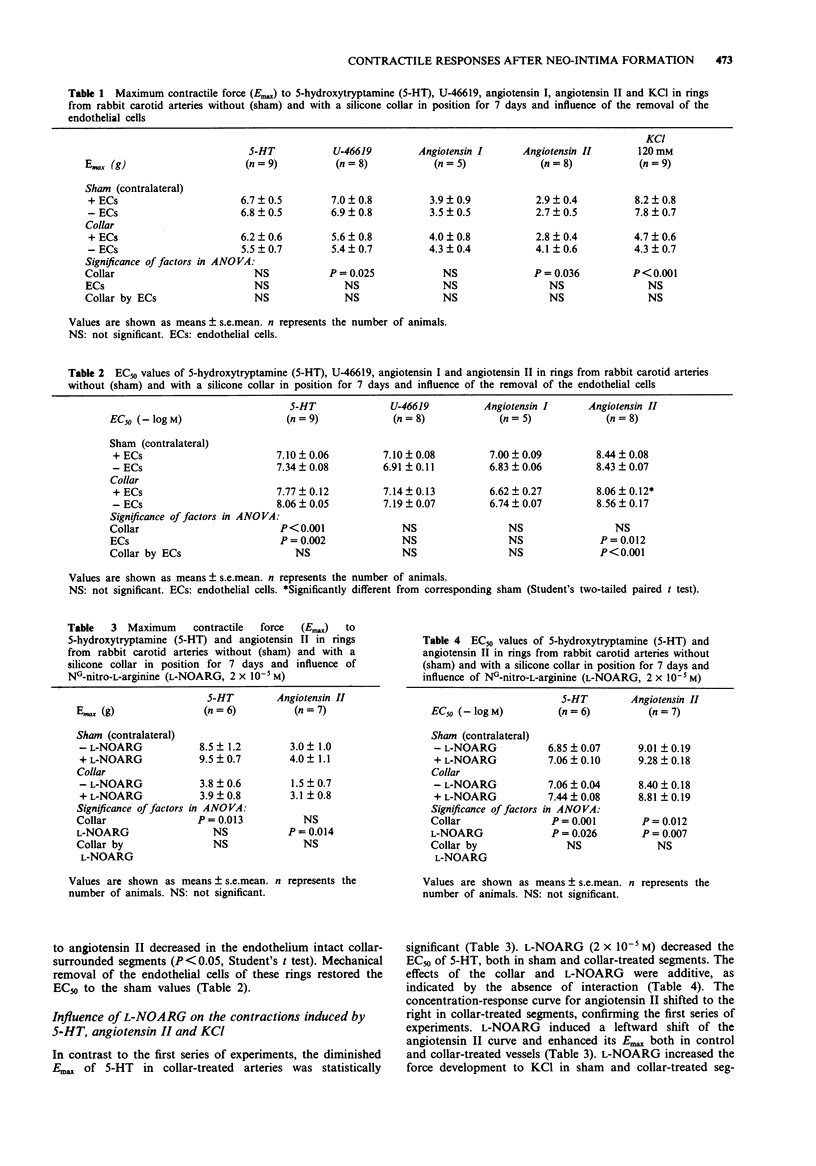
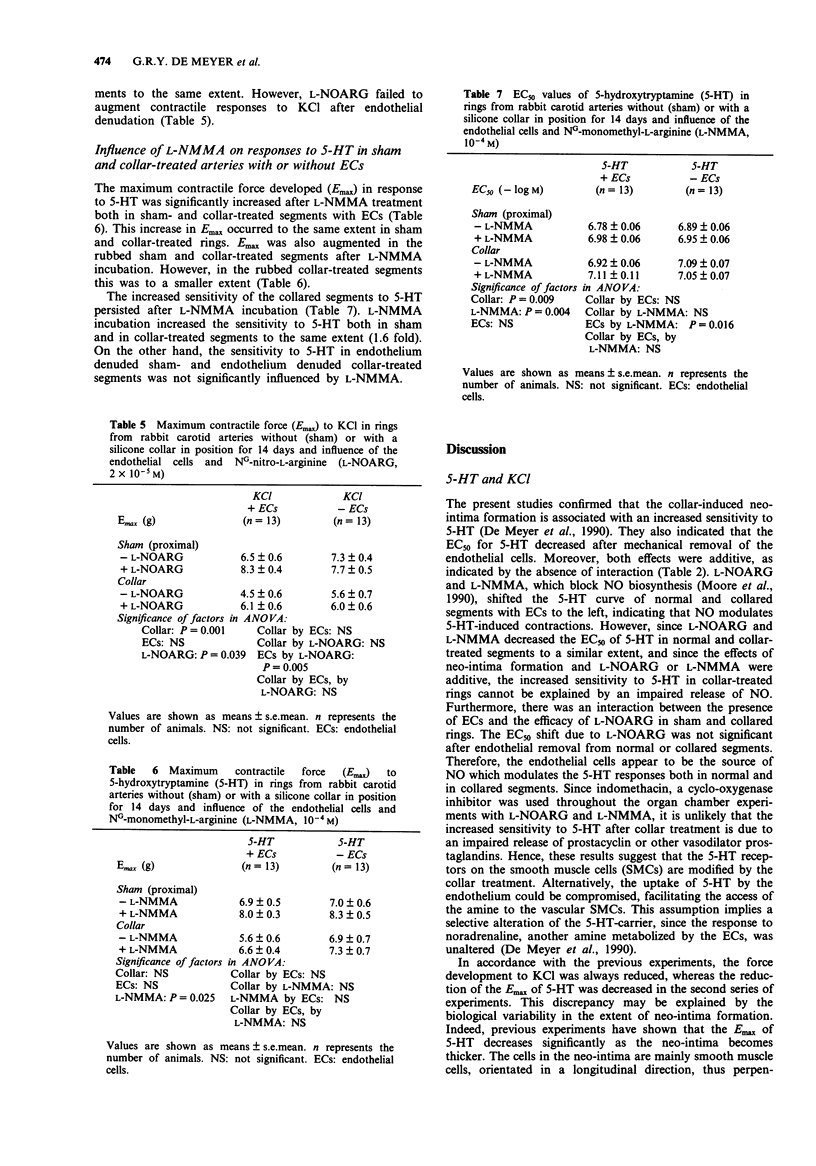


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth R. F., Martin J. F., Honey A. C., Hassall D. G., Beesley J. E., Moncada S. Rapid development of atherosclerotic lesions in the rabbit carotid artery induced by perivascular manipulation. Atherosclerosis. 1989 Apr;76(2-3):257–268. doi: 10.1016/0021-9150(89)90109-3. [DOI] [PubMed] [Google Scholar]
- Bullock G. R., Taylor S. G., Weston A. H. Influence of the vascular endothelium on agonist-induced contractions and relaxations in rat aorta. Br J Pharmacol. 1986 Dec;89(4):819–830. doi: 10.1111/j.1476-5381.1986.tb11187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orléans-Juste P., Dion S., Mizrahi J., Regoli D. Effects of peptides and non-peptides on isolated arterial smooth muscles: role of endothelium. Eur J Pharmacol. 1985 Aug 7;114(1):9–21. doi: 10.1016/0014-2999(85)90515-1. [DOI] [PubMed] [Google Scholar]
- De Meyer G. R., Bult H., Herman A. G. Selective muscarinic alterations of nitric oxide-mediated relaxations by neointima. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S205–S207. doi: 10.1097/00005344-199204002-00058. [DOI] [PubMed] [Google Scholar]
- De Meyer G. R., Bult H., Martin J. F., Van Hoydonck A. E., Herman A. G. The effect of a developing neo-intima on serotonergic and adrenergic contractions. Eur J Pharmacol. 1990 Oct 23;187(3):519–524. doi: 10.1016/0014-2999(90)90380-o. [DOI] [PubMed] [Google Scholar]
- De Meyer G. R., Bult H., Van Hoydonck A. E., Jordaens F. H., Buyssens N., Herman A. G. Neointima formation impairs endothelial muscarinic receptors while enhancing prostacyclin-mediated responses in the rabbit carotid artery. Circ Res. 1991 Jun;68(6):1669–1680. doi: 10.1161/01.res.68.6.1669. [DOI] [PubMed] [Google Scholar]
- Desjardins-Giasson S., Gutkowska J., Garcia R., Genest J. Effect of angiotensin ii and norepinephrine on release of prostaglandins E2 and I2 by the perfused rat mesenteric artery. Prostaglandins. 1982 Jul;24(1):105–114. doi: 10.1016/0090-6980(82)90182-4. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Ryan E. T., Lemke S. M., Bailly D. A., Fox M. K., Schoepp D. D. Endothelium-dependent modulation of angiotensin II-induced contraction in blood vessels. Eur J Pharmacol. 1988 Jan 27;146(1):85–95. doi: 10.1016/0014-2999(88)90489-x. [DOI] [PubMed] [Google Scholar]
- Heistad D. D., Armstrong M. L., Marcus M. L., Piegors D. J., Mark A. L. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984 Jun;54(6):711–718. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- Joly G. A., Schini V. B., Vanhoutte P. M. Balloon injury and interleukin-1 beta induce nitric oxide synthase activity in rat carotid arteries. Circ Res. 1992 Aug;71(2):331–338. doi: 10.1161/01.res.71.2.331. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Cytoskeletal features of normal and atheromatous human arterial smooth muscle cells. Hum Pathol. 1986 Sep;17(9):875–880. doi: 10.1016/s0046-8177(86)80637-2. [DOI] [PubMed] [Google Scholar]
- Kockx M. M., De Meyer G. R., Jacob W. A., Bult H., Herman A. G. Triphasic sequence of neointimal formation in the cuffed carotid artery of the rabbit. Arterioscler Thromb. 1992 Dec;12(12):1447–1457. doi: 10.1161/01.atv.12.12.1447. [DOI] [PubMed] [Google Scholar]
- Manderson J. A., Cocks T. M., Campbell G. R. Balloon catheter injury to rabbit carotid artery. II. Selective increase in reactivity to some vasoconstrictor drugs. Arteriosclerosis. 1989 May-Jun;9(3):299–307. doi: 10.1161/01.atv.9.3.299. [DOI] [PubMed] [Google Scholar]
- Manderson J. A., Mosse P. R., Safstrom J. A., Young S. B., Campbell G. R. Balloon catheter injury to rabbit carotid artery. I. Changes in smooth muscle phenotype. Arteriosclerosis. 1989 May-Jun;9(3):289–298. doi: 10.1161/01.atv.9.3.289. [DOI] [PubMed] [Google Scholar]
- Maseri A., L'Abbate A., Baroldi G., Chierchia S., Marzilli M., Ballestra A. M., Severi S., Parodi O., Biagini A., Distante A. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of "preinfarction" angina. N Engl J Med. 1978 Dec 7;299(23):1271–1277. doi: 10.1056/NEJM197812072992303. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Wang Z. L., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest. 1985 Nov;53(5):556–562. [PubMed] [Google Scholar]
- Oshiro M. E., Paiva A. C., Paiva T. B. Endothelium-dependent inhibition of the use of extracellular calcium for the arterial response to vasoconstrictor agents. Gen Pharmacol. 1985;16(6):567–572. doi: 10.1016/0306-3623(85)90144-2. [DOI] [PubMed] [Google Scholar]
- Saye J. A., Singer H. A., Peach M. J. Role of endothelium in conversion of angiotensin I to angiotensin II in rabbit aorta. Hypertension. 1984 Mar-Apr;6(2 Pt 1):216–221. [PubMed] [Google Scholar]
- Stary H. C., Blankenhorn D. H., Chandler A. B., Glagov S., Insull W., Jr, Richardson M., Rosenfeld M. E., Schaffer S. A., Schwartz C. J., Wagner W. D. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1992 Jan;12(1):120–134. doi: 10.1161/01.atv.12.1.120. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Weisbrod R. M., Cohen R. A. Augmented adrenergic contractions of carotid arteries from cholesterol-fed rabbits due to endothelial cell dysfunction. J Cardiovasc Pharmacol. 1989 Jun;13(6):820–825. doi: 10.1097/00005344-198906000-00003. [DOI] [PubMed] [Google Scholar]
- Toda N. Endothelium-dependent relaxation induced by angiotensin II and histamine in isolated arteries of dog. Br J Pharmacol. 1984 Feb;81(2):301–307. doi: 10.1111/j.1476-5381.1984.tb10079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- WILENS S. L. The nature of diffuse intimal thickening of arteries. Am J Pathol. 1951 Sep-Oct;27(5):825–839. [PMC free article] [PubMed] [Google Scholar]


