Abstract
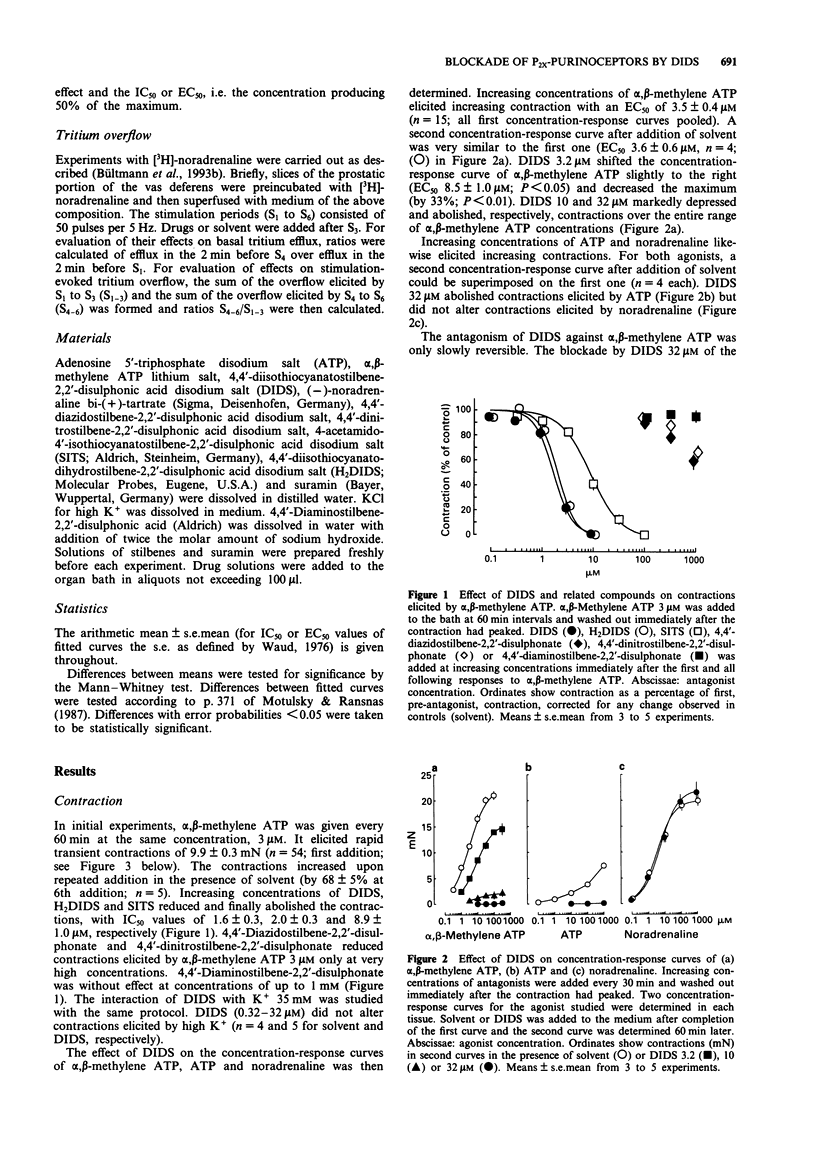
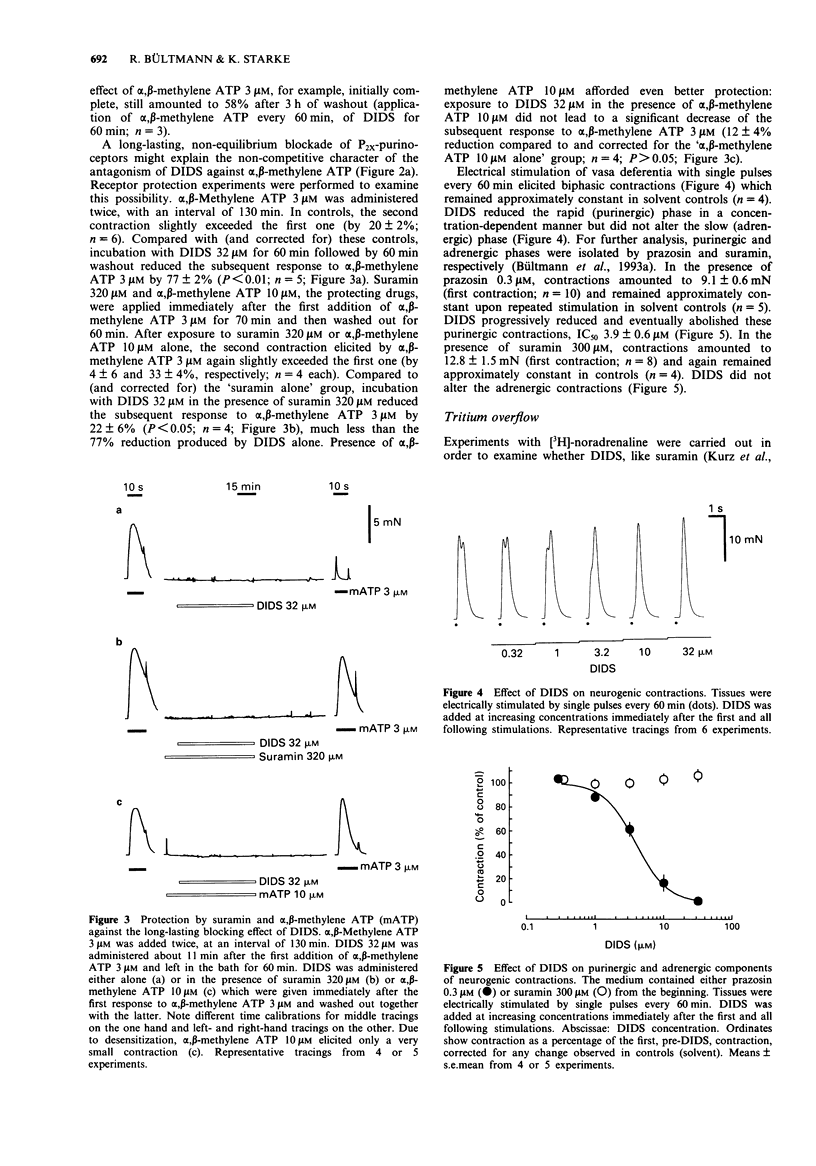
1. The possibility of an antagonist effect of 4,4'-diisothiocyanatostilbene-2,2'-disulphonate (DIDS) at P2X-purinoceptors was studied in rat vas deferens. 2. DIDS reduced contractions elicited by alpha,beta-methylene ATP 3 microM, IC50 1.6 microM, but did not change contractions elicited by K+ 35 mM. DIDS 3.2 microM slightly shifted the concentration-response curve of alpha,beta-methylene ATP to the right and reduced the maximum. DIDS 10 microM markedly decreased and DIDS 32 microM abolished contractions over the entire range of the alpha, beta-methylene ATP concentration-response curve. DIDS 32 microM also abolished contractions elicited by ATP but did not change contractions elicited by noradrenaline. The antagonist effect of DIDS was only slowly reversible. 3. The presence of either suramin 320 microM or alpha,beta-methylene ATP 10 microM during the exposure to DIDS protected the tissue from the long-lasting blocking effect of DIDS. 4. 4,4'-Diisothiocyanatodihydrostilbene-2,2'-disulphonate (H2DIDS) was equipotent with DIDS whereas several analogues in which one or both of the isothiocyanate residues were replaced were less effective or without effect against alpha,beta-methylene ATP. 5. DIDS attenuated the purinergic component of neurogenic contractions elicited by electrical field stimulation, IC50 3.9 microM, but did not change the adrenergic component. 6. It is concluded that DIDS causes a selective, long-lasting, non-equilibrium blockade of P2X-purinoceptors in rat vas deferens. Due to this effect it also selectively blocks the purinergic component of neurogenic contractions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co-transmission. Arch Int Pharmacodyn Ther. 1990 Mar-Apr;304:7–33. [PubMed] [Google Scholar]
- Bültmann R., Starke K. P2-purinoceptor antagonists discriminate three contraction-mediating receptors for ATP in rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1994 Jan;349(1):74–80. doi: 10.1007/BF00178209. [DOI] [PubMed] [Google Scholar]
- Bültmann R., Szabo B., Starke K. Inhibition by ethanol of contractions of rat vas deferens: no evidence for selective blockade of P2X-purinoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1993 May;347(5):527–533. doi: 10.1007/BF00166746. [DOI] [PubMed] [Google Scholar]
- Bültmann R., von Kügelgen I., Starke K. Effects of nifedipine and ryanodine on adrenergic neurogenic contractions of rat vas deferens: evidence for a pulse-to-pulse change in Ca2+ sources. Br J Pharmacol. 1993 Apr;108(4):1062–1070. doi: 10.1111/j.1476-5381.1993.tb13506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol. 1992 Apr;262(4 Pt 1):C803–C827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Chahwala S. B., Cantley L. C. Extracellular ATP induces ion fluxes and inhibits growth of Friend erythroleukemia cells. J Biol Chem. 1984 Nov 25;259(22):13717–13722. [PubMed] [Google Scholar]
- Fedan J. S., Belt J. J., Yuan L. X., Frazer D. G. Contractile effects of nucleotides in guinea pig isolated, perfused trachea: involvement of respiratory epithelium, prostanoids and Na+ and Cl- channels. J Pharmacol Exp Ther. 1993 Jan;264(1):210–216. [PubMed] [Google Scholar]
- Fedan J. S., Lamport S. J. P2-purinoceptor antagonists. Ann N Y Acad Sci. 1990;603:182–197. doi: 10.1111/j.1749-6632.1990.tb37672.x. [DOI] [PubMed] [Google Scholar]
- Fuder H., Muth U. ATP and endogenous agonists inhibit evoked [3H]-noradrenaline release in rat iris via A1 and P2y-like purinoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1993 Oct;348(4):352–357. doi: 10.1007/BF00171333. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Endo J., Kubo R., Kametani F. Inhibitory effects of 4,4'-diisothiocyanostilbene-2,2'-disulfonate (DIDS) on the ADP-stimulated aggregation of gel-filtered bovine blood platelets. Biochem Biophys Res Commun. 1983 Feb 28;111(1):306–311. doi: 10.1016/s0006-291x(83)80152-1. [DOI] [PubMed] [Google Scholar]
- Kurz K., von Kügelgen I., Starke K. Prejunctional modulation of noradrenaline release in mouse and rat vas deferens: contribution of P1- and P2-purinoceptors. Br J Pharmacol. 1993 Dec;110(4):1465–1472. doi: 10.1111/j.1476-5381.1993.tb13986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard N., Marshall R., Sithers A., Spriggs B. Suramin: a selective inhibitor of purinergic neurotransmission in the rat isolated vas deferens. Eur J Pharmacol. 1992 Sep 10;220(1):1–10. doi: 10.1016/0014-2999(92)90004-n. [DOI] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Lechleiter J. D., Cantley L. C., Talamo B. R. Extracellular ATP increases free cytosolic calcium in rat parotid acinar cells. Differences from phospholipase C-linked receptor agonists. Biochem J. 1988 Oct 1;255(1):291–300. [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Ransnas L. A. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987 Nov;1(5):365–374. [PubMed] [Google Scholar]
- Pucéat M., Clément O., Vassort G. Extracellular MgATP activates the Cl-/HCO3- exchanger in single rat cardiac cells. J Physiol. 1991 Dec;444:241–256. doi: 10.1113/jphysiol.1991.sp018875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Talamo B. R., Cantley L. C. Blockade of ATP binding site of P2 purinoceptors in rat parotid acinar cells by isothiocyanate compounds. Biochem Pharmacol. 1993 May 5;45(9):1936–1940. doi: 10.1016/0006-2952(93)90455-6. [DOI] [PubMed] [Google Scholar]
- Stutts M. J., Chinet T. C., Mason S. J., Fullton J. M., Clarke L. L., Boucher R. C. Regulation of Cl- channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. A., Zawisa M. J., Lin X., Hume R. I. A receptor that is highly specific for extracellular ATP in developing chick skeletal muscle in vitro. Br J Pharmacol. 1991 Aug;103(4):1963–1969. doi: 10.1111/j.1476-5381.1991.tb12360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd T. E., Vansterkenburg E. L., Wilting J., Janssen L. H. Recent research on the biological activity of suramin. Pharmacol Rev. 1993 Jun;45(2):177–203. [PubMed] [Google Scholar]
- von Kügelgen I., Kurz K., Starke K. Axon terminal P2-purinoceptors in feedback control of sympathetic transmitter release. Neuroscience. 1993 Sep;56(2):263–267. doi: 10.1016/0306-4522(93)90330-i. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Kurz K., Starke K. P2-purinoceptor-mediated autoinhibition of sympathetic transmitter release in mouse and rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1994 Feb;349(2):125–132. doi: 10.1007/BF00169828. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol Sci. 1991 Sep;12(9):319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]


