Abstract
The effects of immunosuppressive regimens on the outcomes of patients with hematological malignancies undergoing allogeneic stem cell transplantation remain uncertain. We conducted an individual patient data meta-analysis using data from nine randomized trials comparing allogeneic peripheral blood stem cell (PBSCT) transplants to bone marrow (BMT) transplants, focusing on the administration of three vs four doses of methotrexate (MTX) as part of a regimen for graft-versus-host-disease (GVHD) prophylaxis which included cyclosporine. Six trials containing 573 patients prescribed four doses of MTX while three trials containing 534 patients prescribed three doses of MTX. Four doses of MTX conferred a statistically significant survival advantage, resulting in death odds ratio (OR) 0.67 (CI 0.52–0.88) (P=0.0036) for recipients of PBSC compared to BM; with three doses, there was no statistically significant difference. In the four-dose studies relapse rates were 36.6% among recipients of BM compared to 19.2% among recipients of PBSC (P=0.0015). The rates of relapse in the three dose studies were 26% for both PBSC and BM. We hypothesize that the fourth dose of MTX provides extra immunosuppression among BM recipients resulting in a reduced anti-leukemic effect. This hypothesis can only be proved or disproved by a prospective, randomized trial.
Keywords: allogeneic, peripheral blood stem cells, bone marrow, methotrexate
Introduction
Peripheral blood stem cells (PBSC) have replaced bone marrow (BM) as the preferred source of hematopoetic stem cells used for autologous transplantation. Recent surveys indicate that PBSC are used in 50–60% of allogeneic stem-cell transplants.1 The relative effects of allogeneic PBSC transplant vs BM transplant on the outcomes of patients with hematological malignancies are uncertain. In order to address this question, several randomized controlled trials have been conducted. Despite several well designed and executed clinical trials, when taken individually, most of these trials were too small to draw definitive conclusions and, not surprisingly, substantial controversy still remains regarding the impact on graft-versus-host-disease (GVHD), mortality, disease control and other important clinical outcomes.2–4
This is a common situation in health care research and demonstrates the need for a systematic review of the totality of relevant research evidence to determine the relative merits of new interventions and therapies. The ‘gold-standard’ for combining evidence from existing randomized trials is an individual patient data meta-analysis (IPD-MA), in which updated data on each and every participant from each and every relevant trial is centrally collected, processed and analyzed.5,6 The Stem Cell Trialists Collaborative Group have published results of the first analysis comparing global outcomes of randomized studies comparing PBSC to BM.7 The analysis found that PBSC are associated with a decrease in the rates of relapse, which may improve disease-free and overall survival in patients with late-stage disease. The use of PBSC was associated, however, with an increased risk of chronic GVHD.
It is not clear which drugs and schedules for prevention of GVHD are optimum and whether the effects of these drugs differ depending on the stem cell source. Here, we report the second analysis of IPD-MA examining the effect of day 11 methotrexate on the outcomes between HLA-matched, related allogeneic PBSCT and BMT as therapy for hematological malignancies.
Methods
The lead authors from published randomized trials comparing allogeneic PBSC with BM8–18 were contacted and agreed to collaborate and contribute updated individual patient data to this effort. Procedures for the meta-analysis based on the individual patient data have been published in our first report and followed recommended procedures.5–7,19
Statistical methods
Extensive data checking was performed using methods described previously.5,6,19,20 First, data were checked for obvious inconsistencies and amended as necessary through intensive correspondence with the responsible principal investigators. Raw data were also compared with aggregate data in available publications. Particular attention was given to the quality of the randomization procedure used in each trial and the elements of the trials’ quality assessment. This was carried out by checking for any imbalance in accrual between two randomized arms, follow-up and length of follow-ups and the numbers in subgroups.
All comparisons were based on the intention-to-treat principle. Individual patient data allow calculation of required statistics using the exact dates of events, which is more statistically reliable and clinically informative than basing the calculations on proportions alive at a particular point in time.5,20,21 The individual log-rank statistics were combined to give an overall estimate of the effect of PBSCT vs BMT on the outcomes of interest. When information from different trials is combined in this way, the patients in a given trial are compared directly only with other patients5 in the same trial, and not with the patients in another trial.6,20,21 The individual patient data are never pooled in such an analysis. The combination of data from different trials yields an overall estimate of the effect of treatment in all trials, which is then used to calculate reductions in odds of death or other outcomes of interest. All P-values are two-tailed. The results are expressed in such a way that a proportional reduction of a quarter in the annual odds of death might equivalently be described as an odds ratio of 0.75, a hazard ratio of 0.75, an odds reduction of 25%, or a 25% reduction in the death rate.5,21 To test for the difference between overall effect size and the measure of effect from each study a statistical test for heterogeneity was performed across all trials as well as between the subgroups.5 All subgroup analyses were defined a priori.
Differences in the effects between subgroups of patients who were given four doses of MTX vs those who were given three doses, were formally investigated using tests for heterogeneity (interaction) to assess whether the effect size might be different among the studies/subgroups, that is, if observed variability in results is greater than that expected to occur by chance. The main endpoints analyzed were: overall survival, relapse or progression, GVHD, disease-free survival, death in remission and engraftment. Time was calculated from the date of randomization; in the case of acute and chronic GVHD it was calculated from the date of transplant and day + 100 after the transplant, respectively. Disease-free survival was defined as time to death or relapse, whichever occurred first.
A uniform consensus among all trialists was achieved to separate disease in those with ‘good’ prognostic features (chronic myelogenous leukemia (CML) in first chronic phase, acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) in first complete remission, and refractory anemia/refractory anemia with ringed sideroblasts subtypes of myelodysplastic syndromes (MDS)) and ‘poor’ prognostic features (CML in second chronic phase, accelerated phase or blast crisis; AML or ALL, refractory or in greater than first remission; refractory anemia with excess blasts or in transformation subtypes of MDS, multiple myeloma, Hodgkin’s disease, non-Hodgkin’s lymphoma, and idiopathic myelofibrosis).
Results
Trials and patients
While some differences existed among the trials, it was felt that all trials tested similar interventions for similar conditions under similar circumstances to allow combining their results in this meta-analysis.5 Individual patient data from one trial (n=29) were not provided,22 and not included in this analysis. Thus, data on 1107 patients from nine trials were included in the final analysis. There were 6 randomized trials comprised of 573 patients where 4 doses of MTX (days 1, 3, 6 and 11) were prescribed in combination with cyclosporine for prophylaxis of GVHD. In three trials, comprised of 534 patients, MTX was given on days 1, 3 and 6 in combination with cyclosporine. Overall, treatment groups appeared well balanced according to the most important prognostic features (i.e. age, sex, disease type, etc). Characteristics of each trial and the patients included are listed in Table 1.
Table 1.
General characteristics of the randomized clinical trials that compared PBSCT versus BMT for the treatment of hematological malignancies
| Trial (reference) (no.) | Eligibility criteria | GVHD prophylaxisa | Patients characteristics | Disease |
Risk categoriesb |
|
|---|---|---|---|---|---|---|
| Favorable | Unfavorable | |||||
| Saudi Arabia (unpublished) (N = 83) | Age 15–50
Hematologic malignancies |
CSA/MTX (D+1,3,6) | Median age (range): 23 (15–48) | ALL = 18 | ||
| Donor HLA = sibling | AML = 29 | |||||
| Males (%):45 (54.2%) | CML = 28 | 42 | 39 | |||
| MDS = 8 | ||||||
| France (13) (N = 101) | Age <55 years
ALL, AML and CML (1st chronic phase), Donor HLA = sibling |
CSA/MTX (D+1,3,6) | Median age (range): 36 (16–53) | ALL = 19 | ||
| AML = 45 | ||||||
| CML = 37 | 95 | 6 | ||||
| Males (%):53 (52.5%) | MDS = 0 | |||||
| EBMT (12) (N = 350) | Age 16–55 years | CSA/MTX (D+1,3,6) | Median age (range): 38 (17–58) | ALL = 61 | ||
| De novo AML and ALL (in 1st or 2nd remission or in 1st incipient relapse), CML (in chronic or accelerate phase), MDS (except RAEBT) | AML = 126 | |||||
| CML = 152 | ||||||
| Males (%):196 (56.0%) | MDS = 11 | 301 | 38 | |||
| Donor HLA = sibling | ||||||
| Brazil (10) (N = 56) | Age 10–60 years | CSA/MTX (D+1,3,6,11) or CSA/PRED (3 pts at BMT arm) | Median age (range): 31 (7–60) | ALL = 5 | ||
| Hematological malignancies | AML = 11 | |||||
| Donor HLA = sibling | CML = 31 | |||||
| Males (%):38 (67.8%) | MDS = 5 | 37 | 19 | |||
| Others = 4 | ||||||
| Canada (11) (N = 228) | Age 16–65 years | CSA/MTX (D+1,3,6,11) | Median age (range): 45 (19–65) | ALL = 0 | ||
| CML (in chronic or accelerate phase), AML (in remission) and MDS | AML = 83 | |||||
| CML = 109 | ||||||
| Donor HLA = sibling | Males (%):133 (58.3%) | MDS = 36 | 169 | 53 | ||
| Norway (15) (N = 61) | Age 15–60 years
AML, ALL, CML, PMF and MDS |
CSA/MTX (D+1,3,6,11) | Median age (range): 42 (15–55) | ALL = 8 | ||
| Donor HLA = sibling or one mismatched family donor | AML = 24 | |||||
| Males (%):38 (62.3%) | CML = 26 | 42 | 12 | |||
| MDS = 1 | ||||||
| Others = 2 | ||||||
| UK (17) (N = 39) | Age 15–55 years
Any hematological malignancies |
CSA/MTX (D+1,3,6,11) | Median age (range): 37 (22–52) | ALL = 7 | ||
| Donor HLA = sibling | AML = 13 | |||||
| Males (%):29 (74.3%) | CML = 12 | 23 | 16 | |||
| MDS = 2 | ||||||
| Others = 5 | ||||||
| US1 (18) (N = 176) | Age 12–55 years | CSA/MTX (D+1,3,6,11) | Median age (range): 42 (12–56) | ALL = 22 | ||
| Hematological malignancies | AML = 39 | |||||
| Donor HLA = sibling | CML = 58 | 86 | 89 | |||
| Males (%): 122 (69.3%) | MDS = 16 | |||||
| Others = 39 | ||||||
| US2 (28)c (N = 18) | CML
Donor HLA = sibling |
CSA/MTX (D+1,3,6,11) | Median age (range): 46 (19–61) | |||
| CML = 18 | ||||||
| Males (%):12 (66.7%) | 14 | 4 | ||||
Abbreviations: ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; BMT = bone marrow transplantation; Bu = busulfan; CML = chronic myeloid leukemia; CSA = cyclosporine A; Cy = cyclophosphamide; GVHD = graft-versus-host disease; MDS = myelodysplastic syndrome; MTX = methotrexate; NHL = non-hodgkin lymphoma; PBST = peripheral blood stem cell transplantation; PMF = primary myelofibrosis; Pred = prednisone; RAEBT = refractory anemia with excess blast in transformation; TBI = total body irradiation.
The dose of methotrexate used for GHVD prophylaxis was 15 mg/kg in D1 and 10 mg/kg in the rest of the days (D3, D6 and D11). Three patients in the Brazilian trial received prednisone. Initially, intravenous cyclosporine was used in all trials at a dose ranging from 2–5 mg/kg with a subsequent switch to oral administration according to blood levels.
Risk categorization was unavailable for 19 patients.
Seventy-two patients were included in this report, the majority of whom were in reference.18
Distribution of favorable/unfavorable groups: favorable (‘good’ prognosis): (chronic myelogenous leukemia (CML) in first chronic phase, acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) in first complete remission, and refractory anemia/refractory anemia with ringed sideroblasts subtypes of myelodysplastic syndromes (MDS)).
Unfavorable (‘poor’ prognosis): (CML in second chronic phase, accelerated phase or blast crisis; AML or ALL, refractory or in >first remission; refractory anemia with excess blasts or in transformation subtypes of MDS, multiple myeloma, Hodgkin’s disease, non-Hodgkin’s lymphoma, and idiopathic myelofibrosis).
Engraftment
Both neutrophils and platelets engrafted sooner in the PBSCT arm, regardless of whether three or four doses of MTX were prescribed.
The test for interaction between two subgroups was highly significant: χ2 = 11.4; P<0.0008 for platelet engraftment. No such difference was noted for neutrophil engraftment (test for interaction: χ2 = 0.6; P = 0.4).
Acute GVHD
Overall 40% of patients developed grade II–IV acute GVHD and 30% developed grades III–IV acute GVHD. There were no differences in the risks of acute GVHD grades II–IV between recipients of PBSC or BM, irrespective of whether patients were assigned to received day 11 MTX (OR = 1.09, 95% CI 0.82–1.43, P = 0.55) or not (OR = 1.22, 95% CI 0.91–1.63, P = 0.19). Risk of grades III–IV acute GVHD was similar among recipients prescribed day 11 MTX but among patients not assigned day 11 MTX there was a trend to more GVHD with recipients of PBSC (OR 1.50, 95% CI 0.99–2.29, P = 0.058). The test for interaction between subgroups of patients receiving day 11 MTX and those who did not was not significant (for grade II–0-IV, χ2 = 0.3; P<0.6; for grade III–IV, χ2 = 0.3; P = 0.6).
Chronic GVHD
There was a very significant increase in the odds of developing chronic GVHD, both extensive and any stage, in patients treated with PBSC, irrespective of whether patients received day 11 MTX, any stage (OR = 1.43, 95% CI = 1.51–3.23, P<0.016), or did not, any stage (OR = 1.96, 95% CI = 1.45–2.65, P<0.00001) (Figure 1a and b show chronic extensive GVHD). The test for interaction between subgroups of patients receiving day 11 MTX and those who did not was not significant (for chronic extensive GVHD, χ2 = 1.7, P = 0.2).
Figure 1.
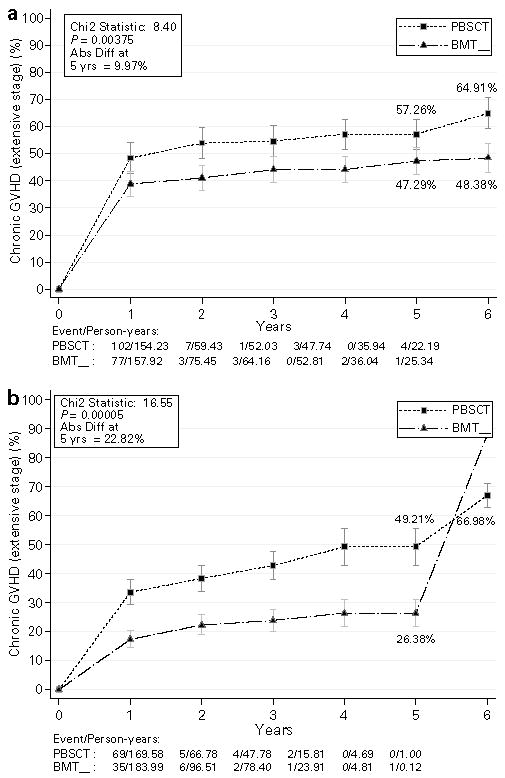
Time-to-event plots showing the absolute risk for development of extensive chronic graft-versus-host disease in the patients with hematologic malignancies who received four doses (a) or three doses (b) of MTX. There is more chronic GVHD of any stage in patients treated with allogeneic PBSC regardless of the MTX doses.
Non-relapse mortality
Among patients assigned to day 11 MTX, non-relapse mortality was 31% in PBSC recipients vs 36% in recipients of BM, P = 0.06. This trend was reversed among patients who did not receive day 11 MTX, at 35% among recipients of PBSC vs 26% among recipients of BM, P = 0.057. The test for interaction between subgroups of patients receiving day 11 MTX and those who did not was significant (for non-relapse mortality, χ2 = 7.0, P = 0.008).
Relapse and relapse-related mortality
Among patients who were assigned to day 11 MTX the risk of relapse at 6 years was 19% in patients receiving PBSC vs 37% in BM recipients, P = 0.0015 (OR 0.54, 95% CI 0.37–0.79) (Figure 2a). In patients who were not assigned to receive day 11 MTX the incidence of relapse was 27% for both recipients of PBSC or BM (Figure 2b). Mortality due to relapse occurred in 24% of patients who received day 11 MTX and BM, compared to 14% in recipients of PBSC, P = 0.018 (OR 0.58, 95% CI 0.37–0.91). The difference in relapse-related mortality among patients who did not receive day 11 MTX was 13% for PBSC and 14% for BM, P = 0.39. The test for interaction between subgroups of patients receiving day 11 MTX and those who did not was significant (for relapse, χ2 = 3.9, P = 0.05).
Figure 2.
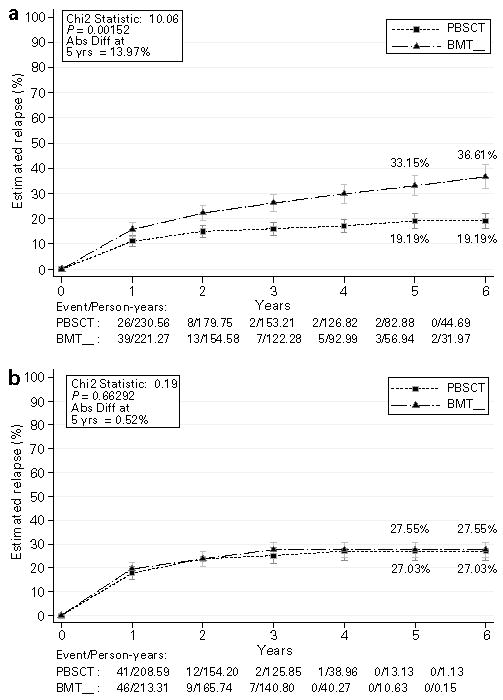
Time-to-event plots showing the absolute risk for development of relapse in the patients with hematologic malignancies who received 4 doses of MTX, all patients (a), and all patients who received only 3 doses of MTX (b).
Disease-free survival
Assignment to day 11 MTX was associated with a significant improvement in disease-free survival (DFS) in recipients of PBSC compared to BM (OR 0.62, 95% CI 0.48–0.80, P = 0.00023). The improvement in DFS with day 11 MTX and PBSC was seen both in patients with early stage disease (OR 0.63, 95% CI 0.44–0.89, P = 0.009) and late stage disease (OR 0.59, 95% CI 0.40–0.86, P = 0.0065). Among the studies that did not use day 11 MTX, there was no difference in DFS between recipients of PBSC vs BM (OR 1.1, 95% CI 0.83–1.45, P = 0.51). The test for interaction between subgroups of patients receiving day 11 MTX and those who did not was significant (for disease-free survival, χ2 = 8.7, P = 0.003).
Survival
Overall survival (OS) was significantly better among recipients of PBSC compared to BM in studies where day 11 MTX was prescribed (OR 0.67, 95% CI 0.52–0.88, P = 0.004). There were no differences in OS between PBSC and BM in studies that did not prescribe day 11 MTX (OR 1.19, 95% CI 0.89–1.60, P = 0.2) (Figure 3a and b). Figure 4 is a tree diagram that summarizes the individual contributions of the nine studies on survival, segregated by whether or not the day 11 methotrexate was prescribed. Note that individually, none of the studies had sufficient power to observe statistically significant differences.
Figure 3.

Survival curves showing the absolute risk reductions in death during the first 6 years of transplant with allogeneic PBSC vs BM depending on whether four doses of MTX (a) or three doses of MTX (b) were given. There was statistically better survival among recipients of PBSC compared to BM in the four dose MTX studies. No statistically significant differences were seen among recipients of PBSC or BM in the three dose trials. Differences in 5-year outcome, together with the standard errors, and two-sided P-value are given in the box.
Figure 4.
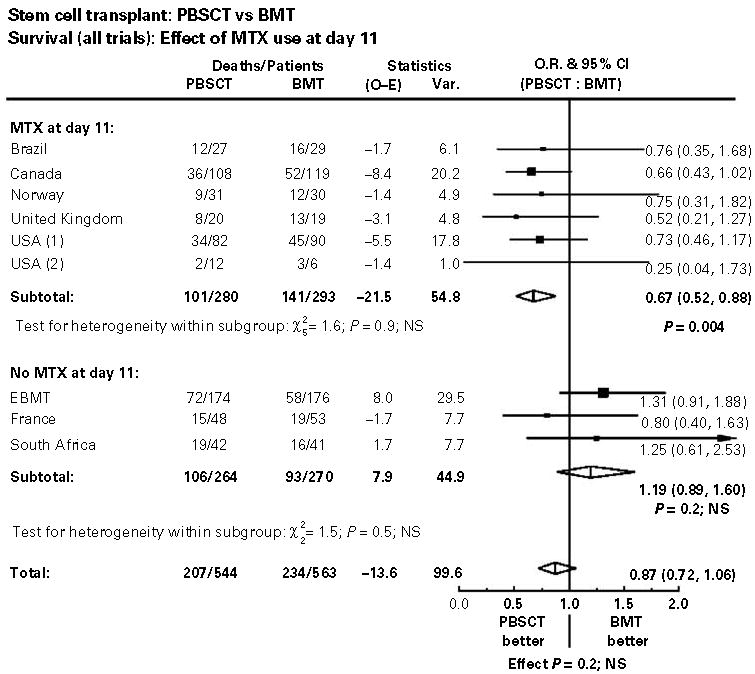
Summary forest plots of survival demonstrating the interaction of day 11 MTX on outcomes. Tests for heterogeneity between MTX and no MTX groups were significant for survival.
The test for interaction between subgroups of patients receiving day 11 MTX and those who did not was significant (for overall survival, χ2 = 8.0, P = 0.005).
Discussion
To address questions regarding the relative advantages and disadvantages of hematopoietic stem cells sources, the stem cell trialists’ collaborative group conducted the first IPD-MA of prospective randomized trials examining transplantation of HLA-matched, related allogeneic PBSC and BM in patients with hematologic malignancies.7 In the first analysis it was found that allogeneic transplants with PBSC were associated with reduced relapse rates and improved disease-free and overall survival primarily in patients with late stage disease. PBSC were also associated with significantly more chronic GVHD.
The present analysis was undertaken primarily to define the relative roles of GVHD prophylaxis when PBSC or marrow are used. This current analysis found that the relative effect of PBSC and BM on disease-free and overall survival was significantly different between the subgroups of trials, defined by whether the administration of day 11 MTX was part of the protocol. This difference was due primarily to a significantly lower rate of relapse and relapse-related deaths among recipients of PBSC in the four dose MTX trials. There were no statistically different rates of transplant-related deaths between recipients of PBSC or BM, regardless of whether three or four doses of MTX were prescribed, although trends for non-relapse mortality were reversed; favoring PBSC when day 11 MTX was given and BM when day 11 MTX was not. In trials prescribing only three doses of MTX there were no differences in relapse or survival between PBSC and BM. The differences in relapse rates between the four dose MTX recipients of BM or PBSC could be due to a lower relapse rate among PBSC recipients, a higher relapse rate in recipients of BM or a combination of both. Unfortunately, the meta-analysis does not allow direct comparisons of relapse rates of either BM recipients or PBSC recipients between the three dose MTX and four dose MTX studies. This is in part due to differences in the mix of risk factors between the different studies and because it is important to preserve randomization of patients within each study.
The combination of MTX and cyclosporine has been widely adopted as the preferred regimen for prevention of GVHD due to its superior efficacy compared to single agent MTX or CSP.23 The close interactions of GVHD prophylaxis, GVHD and relapse after allogeneic BM transplant are well known. Some, but not all studies have identified an association between more intensive immunosuppressive regimens and higher rates of relapse.24 In a long-term follow-up of a randomized trial of MTX and cyclosporine versus cyclosporine alone in patients undergoing allogeneic BM transplant, Storb et al.23 found increased leukemia relapses in patients with acute non-lymphocytic leukemia, but not in patients with CML. In an analysis of 199 HLA identical sibling donor transplants, Nordlander et al.25 found that combination GVHD prophylaxis with MTX and cyclosporine was associated with a greater risk of relapse (HR 2.56, OR 1.22–5.37, P = 0.01) compared to single agent MTX or cyclosporine. A retrospective study from the International Bone Marrow Transplant Registry found no differences in relapse rates among patients with leukemia receiving cyclosporine alone or combined with MTX. These studies included patients with differing MTX schedules and also included patients who had received prednisone as part of GVHD prophylaxis, and this mixture of patients could have easily obscured the effects of MTX dosing.26 In our study, the MTX schedules, whether they were prescribed for 3 or 4 days were uniform across the trials as shown in Table 1.
The reasons why four doses of MTX might have such a differential effect on outcomes that are dependent on stem cell source are not entirely clear. It is well established that MTX containing regimens slow the rates of neutrophil and platelet engraftment. In the present analysis, neutrophil and platelet engraftment occurred more rapidly and with a higher percentage of patients achieving engraftment in recipients of PBSC regardless of whether or not the fourth dose of MTX was given.
The administration of the fourth dose of MTX may provide critical immunosuppression by causing apoptosis of rapidly dividing lymphocytes at the time of early engraftment. This could impair the antileukemia effects of donor lymphocytes. More recent studies suggest that low dose MTX may prevent activation of T cells rather than apoptosis.27 We speculate that these effects could be more pronounced in recipients of BM compared to PBSC due to the 1 log greater numbers of T cells. In addition, the differential sensitivity of specific diseases to GVL effects are well known.23 As the IPD-MA contained patients with a variety of different diseases, the GVL effect may be more or less apparent depending on the mix of diagnoses within a given study.
Our analysis should be interpreted within the context of the extreme logistical difficulties associated with performing large randomized trials in allogeneic transplantation. As hematological diseases are rare, and transplant numbers are relatively low even in large centers, most trials had to enroll patients with a variety of hematological malignancies containing a mix of standard risk and high-risk patients. However, by putting together the results of all existing trials we were able to increase the power of the analysis.
To date, no randomized studies have examined whether the administration of three or four doses of MTX in combination with cyclosporine affects any outcomes in recipients of allogeneic PBSC or BM. The present report suggests that further prospective studies to test these observations are warranted.
Acknowledgments
Funding source: Main funding for this project was provided by the NHI/NHLBI Grant # 1R01HL71650–01 (Drs Djulbegovic and Bensinger) and in part by The Jose Carreras Foundation Against Leukemia, NCI CA18029, CA18221 (Dr Bensinger), the Swiss National Research Foundation, and the French Ministry of Health (Programme Hospitalier de Recherche Clinique 1996) (Dr Gratwohl) and a grant from the Ligue Nationale de Lutte Contre le Cancer (Dr Blaise).
Appendix
Members of Stem Cell Trialists’ Collaborative Group (in alphabetical order):
Mahmoud al-Jurf – Department of Medicine, King Faisal Specialist Hospital & Research Center, Riyadh, Kingdom of Saudi Arabia
Claudio Annasetti, H. Lee Moffitt Cancer Center & Research Institute, University of South Florida, Tampa, USA
Jane F. Apperley – EBMT – Department of Haematology, Faculty of Medicine, Imperial College, Hammersmith Hospital, London, United Kingdom
Roy Baynes – Amgen, Thousand Oaks, California, USA
William I. Bensinger – Fred Hutchinson Cancer Research Center, Seattle, Washington, USA
Didier Blaise – France – Unite de Transplantation et de Therapie Cellulaire (UTTC), Institut Paoli-Calmettes, Marseille and French society of transplantation (SFGM-TC), France
Mike Clarke – UK Cochrane Center
Ed Colcol – Department of Oncology, Section of Adult Hematology/BMT, King Faisal Specialist Hospital and Research Center Health Care System, Riyadh, Saudi Arabia
Jan J Cornelissen – Department of Hematology, Daniel den Hoed Cancer Center, Rotterdam, The Netherlands
Stephen Couban – Canadian Bone Marrow Transplant Group, Department of Medicine, Dalhousie University and Queen Elizabeth II Health Sciences Centre, Halifax, NS, Canada
Corey Cutler – Dana Farber Cancer Institute, Boston, MA, USA
Benjamin Djulbegovic – H. Lee Moffitt Cancer Center & Research Institute, University of South Florida, Tampa, USA (PI for the NIH/NHLBI grant # 1R01HL71650-01)
Alois Gratwohl – Kantonsspital, Basel, Switzerland
Dag Heldal – Medical Department, Rikshospitalet University Hospital, 0027 Oslo, Norway
Robert K Hills University of Birmingham Clinical Trials Unit, University of Birmingham, Birmingham, UK
Iztok Hozo – Department of Mathematics, Indiana University, Gary, IN, USA
Mathieu Kuentz, MD; Department of Hematology, Hopital Henri Mondor, Creteil and French society of transplantation (SFGM-TC), France
Ambuj Kumar – H. Lee Moffitt Cancer Center & Research Institute, University of South Florida, Tampa, USA
Jeffrey H. Lipton-Princess Margaret Hospital, Toronto, Canada
Eliana CM Miranda- Bone Marrow Transplantation Unit, State University of Campinas, Brazil
Mohamad Mohty – France – Unite de Transplantation et de Therapie Cellulaire (UTTC), Institut Paoli-Calmettes, Marseille Marseille and French society of transplantation (SFGM-TC), France
James Matcham, Amgen, Thousand Oaks, California, USA
James Morton – Bone Marrow Transplant Unit, Royal Brisbane Hospital, Herston, Australia
Tony Panzarella – Canadian Bone Marrow study group – Princess Margaret Hospital, Toronto, Ontario, Canada
Ray Powles – Royal Marsden Hospital, Sutton, UK
Sue Richards, Clinical Trial Service Unit, Oxford University, UK
Entezam Sahovic – Department of Oncology, Section of Adult Hematology/BMT, King Faisal Specialist Hospital and Research Center Health Care System, Riyadh, Saudi Arabia
Norbert Schmitz – Abteilung Hämatologie, AK St. Georg, Germany
David R. Simpson, North Shore Hospital, Takapuna, Auckland, New Zealand
Bhawna Sirohi – Royal Marsden Hospital, Sutton, UK
Heloisa P Soares – H. Lee Moffitt Cancer Center & Research Institute, University of South Florida, Tampa, USA
Carmino A de Souza – Bone Marrow Transplantation Unit, State University of Campinas, Brazil
B Van der Holt – Department of Hematology, Daniel den Hoed Cancer Center, Rotterdam, The Netherlands
Afonso C Vigorito – Bone Marrow Transplantation Unit, State University of Campinas, Brazil
Keith Wheatley University of Birmingham Clinical Trials Unit, University of Birmingham, Birmingham, UK
References
- 1.Gratwohl A, Baldomero H, Horisberger B, Schmid C, Passweg J, Urbano-Ispizua A. Current trends in hematopoietic stem cell transplantation in Europe. Blood. 2002;100:2374–2386. doi: 10.1182/blood-2002-03-0675. [DOI] [PubMed] [Google Scholar]
- 2.Korbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98:2900–2908. doi: 10.1182/blood.v98.10.2900. [DOI] [PubMed] [Google Scholar]
- 3.Bensinger WI, Clift R, Martin P, Appelbaum FR, Demirer T, Gooley T, et al. Allogeneic peripheral blood stem cell transplantation in patients with advanced hematologic malignancies: A retrospective comparison with marrow transplantation. Blood. 1996;88:2794–2800. [PubMed] [Google Scholar]
- 4.Bensinger WI, Deeg HJ. Blood or marrow? (Commentary) . Lancet. 2000;355:1199–1200. doi: 10.1016/s0140-6736(00)02080-8. [DOI] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists Collaborative Group. Poly-chemotherapy for early breast cancer: an overview of the randomized trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 6.Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med. 1995;14:2057–2079. doi: 10.1002/sim.4780141902. [DOI] [PubMed] [Google Scholar]
- 7.Stem Cell Trialists’ Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of 9 randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood. 2001;98:3186–3191. doi: 10.1182/blood.v98.12.3186. [DOI] [PubMed] [Google Scholar]
- 9.Vigorito AC, Azevedo WM, Marques JF, Azevedo AM, Eid KA, Aranha FJ, et al. A randomised, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of haematological malignancies. Bone Marrow Transplant. 1998;22:1145–1151. doi: 10.1038/sj.bmt.1701510. [DOI] [PubMed] [Google Scholar]
- 10.Vigorito AC, Marques Junior JF, Aranha FJ, Oliveira GB, Miranda EC, de Souza CA. A randomized, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of hematologic malignancies: an update. Haematologica. 2001;86:665–666. [PubMed] [Google Scholar]
- 11.Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–1531. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761–767. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 13.Blaise D, Kuentz M, Fortanier C, Bourhis JH, Milpied N, Sutton L, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Société Française de Greffe de Moelle. J Clin Oncol. 2000;18:537–571. doi: 10.1200/JCO.2000.18.3.537. [DOI] [PubMed] [Google Scholar]
- 14.Cornelissen JJ, van der Holt B, Petersen EJ, Vindelov L, Russel CA, Hoglund M, et al. A randomized multicenter comparison of CD34(+)-selected progenitor cells from blood vs from bone marrow in recipients of HLA-identical allogeneic transplants for hematological malignancies. Exp Hematol. 2003;31:855–864. doi: 10.1016/s0301-472x(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 15.Heldal D, Tjonnfjord GE, Brinch L, Albrechtsen D, Egeland T, Steen R, et al. A randomised study of allogeneic transplantation with stem cells from blood or bone marrow. Bone Marrow Transplant. 2000;25:1129–1136. doi: 10.1038/sj.bmt.1702422. [DOI] [PubMed] [Google Scholar]
- 16.Clarke M, Stewart L. Obtaining individual patient data from randomised controlled trials. In: Egger M, Smith GD, Altman DG, editors. Systemic Reviews in Health Care Meta-analysis in Context. London: BMJ; 2001. [Google Scholar]
- 17.Powles R, Mehta J, Kulkarni S, Treleavan J, Millar B, Marsden J, et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet. 2000;355:1231–1237. doi: 10.1016/S0140-6736(00)02090-0. [DOI] [PubMed] [Google Scholar]
- 18.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 19.Sahovic E. Allogeneic peripheral blood vs bone marrow transplant in hematologic malignancies: a randomized controlled trial. Personal communication. 2002 [Google Scholar]
- 20.Myeloma Trialists’ Collaborative Group. Interferon as therapy for multiple myeloma: an individual patient data overview of 24 randomized trials and 4012 patients. Br J Haematol. 2001;113:1020–1034. doi: 10.1046/j.1365-2141.2001.02857.x. [DOI] [PubMed] [Google Scholar]
- 21.Early Breast Cancer Trialists’ Collaborative Group. Poly-chemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 22.Mahmoud H, Fahmy O, Kamel A, Kamel M, El Haddad A, El Kadi D. Peripheral blood vs bone marrow as a source for allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:355–358. doi: 10.1038/sj.bmt.1701906. [DOI] [PubMed] [Google Scholar]
- 23.Storb R, Deeg HJ, Pepe M, Appelbaum FR, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: Long-term follow-up of a controlled trial. Blood. 1989;73:1729–1734. [PubMed] [Google Scholar]
- 24.Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–1728. [PubMed] [Google Scholar]
- 25.Nordlander A, Mattsson J, Ringden O, Leblanc K, Gustafsson B, Ljungman P, et al. Graft-versus-host disease is associated with a lower relapse incidence after hematopoietic stem cell transplantation in patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2004;10:195–203. doi: 10.1016/j.bbmt.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Horowitz MM, Gale RP, Sondel PM, Kersey J, Kolb HJ, Rimm AA, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 27.Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clinical Immunology. 2005;114:154–163. doi: 10.1016/j.clim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Oehler VG, Radich JP, Storer BS, Blume KG, Chauncey T, Clift R, et al. Randomized trial of allogeneic related bone marrow transplantation versus peripheral blood stem cell transplantation for chronic myeloid leukemia. Biol Blood Marrow Transplant. 2005;11:85–92. doi: 10.1016/j.bbmt.2004.09.010. [DOI] [PubMed] [Google Scholar]


