Abstract
Background
Telemonitoring, the use of communication technology to remotely monitor health status, is an appealing strategy for improving disease management.
Methods
We searched Medline databases, bibliographies, and spoke with experts to review the evidence on telemonitoring in heart failure patients.
Results
Interventions included: telephone-based symptom monitoring (n=5), automated monitoring of signs and symptoms (n=1), automated physiologic monitoring (n=1). Two studies directly compared effectiveness of 2 or more forms of telemonitoring. Study quality and intervention type varied considerably. Six studies suggested reduction in all-cause and heart failure hospitalizations (14% to 55% and 29% to 43%, respectively) or mortality (40% to 56%) with telemonitoring. Of the 3 negative studies, 2 enrolled low-risk patients and patients with access to high quality care while 1 enrolled a very high risk Hispanic population. Studies comparing forms of telemonitoring demonstrated similar effectiveness. However, intervention costs were higher with more complex programs ($8383 per patient per year) versus less complex programs ($1695 per patient per year).
Conclusions
The evidence base for telemonitoring in heart failure is currently quite limited. Based on the available data, telemonitoring may be an effective strategy for disease management in high-risk heart failure patients.
INTRODUCTION
Telemonitoring, the use of communication technology to monitor patients’ clinical status, is gaining attention as a strategy to improve the care of patients with chronic disease. By allowing clinical data to be collected without the need for face-to-face contact with patients, telemonitoring can make care more accessible for patients and has the potential to improve outcomes. The Institute of Medicine’s endorsement of this approach is evident, as the first of its 10 rules for redesigning the health care system outlined in the report, “Crossing the Quality Chasm” (1) is “Patients should receive care whenever they need it and in many forms, not just face-to-face visits.”
Telemonitoring holds particular promise for patients with heart failure, who often experience deterioration in their health status with an increase in weight and symptoms over a period of days and weeks before presenting to medical attention and requiring hospitalization. A system of frequent monitoring could alert clinicians to the early signs and symptoms of decompensation, providing the opportunity for intervention before patients become severely ill and require hospitalization. Moreover, patients’ participation in communicating information about their weight and health status on a daily basis could have a favorable effect on their health behaviors, including adherence to medical recommendations.
The importance of telemonitoring is highlighted by national efforts to disseminate this approach. Telemonitoring is being used to care for patients with a variety of chronic conditions in the Veterans Affairs healthcare system (2,3) and in some managed care settings (4). The Centers for Medicare and Medicaid Services is implementing widespread demonstration projects that mandate the use of remote monitoring technologies to enable the exchange of pertinent clinical information, such as vital signs and symptoms (5). Up to 300,000 patients with heart failure, complex diabetes and chronic obstructive pulmonary disease will be enrolled, and the interventions may eventually be implemented nationally. Previous reviews of telemonitoring in heart failure (6,7) have not provided an in-depth exploration of the details of each intervention, a necessary step in understanding which components make a program effective. Unless the following critical issues are made transparent, telemonitoring will remain poorly understood:
What did the telemonitoring program consist of?
Who was responsible for acting on the information obtained?
How was the intensity and duration of the program modulated to meet the clinical needs of patients?
What are the costs of implementing the telemonitoring program and what effect did the program have on health care costs?
These reviews also limited their analyses to studies of rather sophisticated forms of telemonitoring: studies in which the intervention was based purely on telephone monitoring were not included. We sought to extend the results of these prior reviews of telemonitoring in heart failure by performing an in-depth examination of a wide range of telemonitoring interventions with the addition of several recently published studies not previously analyzed (8–10).
METHODS
We chose to group studies according to the primary mode of the telemonitoring intervention as this is a defining feature. The primary mode of intervention (telephone-based symptom monitoring, automated monitoring of signs and symptoms, automated physiologic monitoring, and comparisons of 2 or more methods) thus serves as the basis for the sub-group analyses in the Results section.
Evidence Acquisition
Studies for this review were obtained from Ovid MEDLINE and EMBASE searches (performed August 20, 2006 with the assistance of a professional librarian) of English language literature from 1966 to August 2006. We used the MESH term heart failure, congestive and text word variations combined with any of the following MESH terms and their text word variations: telemedicine, telecommunication, telecare, telehealth, telenursing, telemanagement, telecardiology, teleconsult, telediagnosis, remote diagnosis, remote consult, remote monitor. This search resulted in 97 articles.
Inclusion Criteria
In selecting studies for inclusion in this review, we required that studies use home telemonitoring for adult patients with heart failure and that they assess hospitalization or mortality rates. We also required a randomized study design. In an effort to obtain data that may be available but not published, we contacted primary study authors if outcomes of interest were not available in the published report.
Telemonitoring spans a gamut of modalities, ranging from simple telephone systems to sophisticated systems of physiologic monitoring. Although some authors have chosen to narrowly define it as “telephone plus” interventions (6,7), the Institute of Medicine defines telemonitoring more broadly as “the use of electronic information and communications technologies to provide and support health care when distance separates the participants.” (11) As there is no clear rationale for excluding telephone systems, they are included here. For the purposes of this review, we excluded studies that had in-person, face-to-face components to the monitoring intervention, since our goal was to synthesize the evidence for remote monitoring of heart failure patients.
Data Extraction
One of the investigators (SIC) reviewed all abstracts retrieved by the literature search. Abstracts needed to appear potentially relevant to the study area and 63 were deemed not relevant. Two investigators (SIC and CP) then independently assessed each article to determine its appropriateness for inclusion. We searched the bibliographies of all studies retrieved from our original search for additional relevant work, also including articles suggested by experts in the field. This search strategy yielded 9 studies that were included in this review. Given the heterogeneity among studies in nature of intervention, study population, duration of follow-up, and outcomes assessed, we made an a-priori decision not to pool results from individual studies.
Quality Assessment
Quality assessment of telemonitoring trials is challenging as many of the widely used quality scales emphasize study participant blinding, which is not possible in this type of trial (12). In assessing quality of trials in this review, we used criteria suggested by Juni et al (13) as well as those recommended by the York Centre for Reviews and Dissemination (14). These methods yielded a total of 6 criteria by which we assessed the quality of studies.
RESULTS
Of the 9 studies included in this review, 2 were conducted at a single site and 8 were conducted within the United States.
Evidence Synthesis
Telephone-Based Symptom Monitoring
The 5 studies in this category all employed an intervention based upon symptom monitoring by nurses via live one-on-one telephone calls with patients. Despite having this basic feature in common, the studies differ widely in design, including who was responsible for initiating management changes, the complexity of the intervention and patient population enrolled.
In the DIAL trial (10), patients received telephone-based monitoring and education from centralized nurses trained in the management of heart failure. The intervention was based on 5 objectives: symptom monitoring, adherence to diet and medications, optimal fluid status, and daily physical activity. Nurses used a software program to determine call frequency (calls occurred every 2 weeks for 8 weeks then at a variable frequency). If clinical deterioration was detected, the nurses used a standardized algorithm to adjust diuretic doses or recommend urgent medical visits. There was a 20% relative risk reduction in the primary, combined outcome of heart failure hospitalizations and death (95% Confidence Interval (CI) 3–34), which was largely due to reductions in heart failure hospitalization.
The intervention employed by Dunagan et al (17) was similar to the DIAL intervention. Centralized nurses trained in the management of heart failure made calls to promote self-management skills. Though the intervention was telephone-based, 20 patients also received home visits for additional support initially. During the calls (which occurred weekly for 2 weeks and then according to individual patients’ needs), nurses also screened for evidence of heart failure decompensation and, based on clinical judgment, could advise the patients to take supplemental diuretic doses. A 47% reduction was seen in the primary outcome of all-cause hospitalization or emergency room visits (95% CI 0.36–0.80). Of note, the investigators prematurely terminated the planned enrollment of 500 patients after 151 patients due to logistical and financial constraints.
In the study by Riegel et al, registered nurses monitored patients’ clinical status and educated them about heart failure. (16). The frequency of calls was determined by nurses using decision support software. The software also set priorities for patient education and was designed to emphasize adherence and recognition of worsening illness. While symptom monitoring was performed, clinical management was deferred to patients’ physicians, who were sent reports on patients’ status. A 50% reduction was seen in the primary outcome of heart failure hospitalizations (95% CI 0.35–0.73). The program was found to be cost-saving: the average cost of acute care was $1000 less per patient in the intervention group (compared with the usual care group) over 6 months and the 6 month intervention cost was $443 per patient.
In a separate study also by Riegel et al, (18) the same intervention used in this group’s prior study (16) was implemented in a group of Hispanics of Mexican origin who were hospitalized with heart failure. The intervention was refined to be culturally appropriate, with more emphasis on contacts with the family. The study sample was poorly educated (78% less than high school education) and mostly unacculaturated into U.S. society (55%). In this study, no significant differences were found in the primary outcome of heart failure hospitalizations
In the DeBusk study (9) centralized nurses performed symptom monitoring, heart failure education and pharmacologic management. Nurse case-managers also initiated and regulated medications for heart failure according to study protocol. In addition to scheduled calls (which occurred weekly for 6 weeks and then at a decreased frequency), nurse judgment was used to determine need for supplemental calls and to tailor content to individual patient needs. This study is notable as one of the few in the heart failure disease management literature to demonstrate no benefit: the relative risk (RR) for heart failure hospitalization was 0.91 (95% CI 0.71–1.16) and the RR for all-cause hospitalization was 0.98 (95% CI 0.76–1.27).
Studies in this category are recent, large-scale and of high quality. Three of the 5 trials demonstrated decreased heart failure hospitalization rates and 2 demonstrated decreased all-cause hospitalizations. Eligibility proportions range from 0.20–0.64 with the DIAL trial having the highest proportion. Only the study done in 2002 by Riegel (16) included an economic analysis comparing cost with financial savings and demonstrated cost benefit of the program. While all studies in this category were based on live telephone monitoring by nurses, the content, complexity and implementation of the interventions varied widely.
Automated Monitoring of Signs and Symptoms
This study is based on automated monitoring of patients’ signs and symptoms. Monitoring is accomplished by patients entering data into an electronic communication device with information then downloaded into a secure website to be reviewed by clinicians at a later time.
In the Weight Monitoring in Heart Failure (WHARF) trial, Goldberg et al enrolled recently hospitalized patients followed at heart failure specialty clinics to determine the effects of twice daily electronic home monitoring of symptoms and weight on hospital readmission (19). The system consisted of an electronic scale placed in patients’ homes with a monitor for patients to answer questions about symptoms. The home-based system was linked to a computerized database monitored by trained cardiac nurses employed by Alere®. Cardiac nurses reviewed data, but responsibility for acting upon the information rested solely with patients’ physicians, who were updated regularly about patients’ status. No difference in the primary endpoint of all-cause hospitalization rates was observed (RR 0.85 (95% CI 0.6–1.25)). Interestingly, a 56% reduction (95% CI 0.22–0.85) in mortality (a pre-specified secondary endpoint) was observed with use of the telemonitoring intervention.
The WHARF trial demonstrated no benefit in the primary outcome of hospitalization rates. This study was relatively recent but did not include economic analysis. Despite the proliferation of systems to support home-based, automated monitoring, there is relatively little evidence to support their effectiveness.
Automated Physiologic Monitoring
Collection of physiologic data can take a variety of forms. Benatar et al compared the effectiveness of daily self-monitoring of weight, blood pressure, heart rate and oxygen saturation with home nurse visits (20). Physiologic monitoring was performed by patients in their homes, with the information transmitted to a secure internet site for review by patients’ cardiologists. Home visits were scheduled 3 times per week during the first week, decreasing in frequency thereafter to 1 visit by the fourth week and further visits on an “as-needed basis.” The content of these visits included discussion of diet, symptom recognition and adherence with medications. There was a 40% reduction (95% CI 0.45–0.82) in heart failure admissions among the telemonitored group compared with the nurse home visit group. The daily cost of the telemonitoring intervention was reported to be $2.87 and the 6 month cumulative readmission charges were $223,638 in the telemonitoring group and $500,343 in the group receiving nurse visits.
This study of automated physiologic monitoring demonstrated reduction in heart failure hospitalizations and included analyses suggesting that the intervention may be cost-beneficial. This study met fewer of our quality criteria (2) than studies in the categories discussed above.
Comparisons of 2 or More Methods of Telemonitoring
Given the diversity of telemonitoring interventions, studies that directly compare the effectiveness of 2 or more modalities are quite useful. Cleland et al enrolled 426 patients recently admitted for heart failure in 3 European countries to compare the effectiveness of physiologic monitoring versus nurse telephone support (8). Physiologic monitoring was performed twice daily in patients’ homes using automated devices including an electronic scale, sphygmomanometer, and an electrocardiogram. Each device was linked to a central web server for review by study nurses. The nurses could make short-term changes in therapy or work through patients’ primary physicians if long-term therapeutic changes were needed. The intervention in the nurse telephone support arm consisted of monthly calls by a heart failure specialist nurse to assess symptoms and medications. Nurses offered advice to patients and provided feedback to patients’ primary physicians. While outcomes were improved in both intervention arms compared with usual care, no between-group differences in the primary outcome of days lost to death or hospitalization were seen in the group receiving physiologic monitoring and the group receiving telephone support (12.7% versus 15.9% respectively).
Jerant et al randomized 37 patients to receive: video conferencing with an integrated stethoscope; nursing support by telephone; and usual care (15). The frequency of each intervention was tailored to meet individual patients’ needs, with the overall goal of monitoring signs and symptoms as well as patient education. Video conferencing employed a camera to allow observation of respiratory effort and leg edema and an integrated stethoscope applied to standard heart and lung auscultation points. Management was deferred to patients’ own physicians, who were sent summaries and recommendations. While 6-month heart failure readmission charges were higher in the usual care group compared with the 2 intervention groups, no differences were seen between the video conferencing and telephone support groups ($5850 versus $7320 respectively). Due to small sample size, relative risks could not be calculated to compare admissions and mortality between groups.
The 2 studies comparing 2 or more methods of telemonitoring varied in interventions studied, size and quality – one met all 6 quality criteria, and the other met 4. Although these studies found telemonitoring to be beneficial compared with usual care, various forms of telemonitoring appeared equivalent when tested against each other in these two studies. This finding is striking given that the forms of telemonitoring within each study were quite different in technical sophistication, intensity and cost. The study by Jerant et al included economic analyses, demonstrating both forms of telemonitoring intervention to be cost beneficial. Of note, no incremental benefit was seen with the far more costly video conferencing (1-year cost $7487, inflation-adjusted cost in 2006 United States dollars $8383) compared with telephone support (1-year cost $1514, inflation-adjusted cost in 2006 United States dollars $1695). With a total sample size of only 37 participants, however, it is difficult to draw conclusions about the relative effectiveness of the interventions.
DISCUSSION
In this review, we have summarized the evidence for interventions based solely on telemonitoring without any face-to-face component. Based upon this review it appears that the literature is in evolution and confounded by several factors. Compared with other forms of heart failure disease management, there are few large, high quality trials that can be used to guide policy regarding implementation of telemonitoring, and no data about long-term sustainability of these programs. High quality data regarding the effectiveness of automated forms of telemonitoring are especially scarce, despite the proliferation of private companies offering such services and the potential benefit of this form of telemonitoring in settings with limited personnel. Rigorous cost-effectiveness analyses with reasonably long-term horizons are scarce and detailed, prospective cost analyses should be included in future studies of HF disease management in general, and telemonitoring interventions in particular. Without a clear understanding of the economic implications of telemonitoring interventions, it will be difficult to establish informed national policies regarding reimbursement for these programs.
Understanding precisely what makes some telemonitoring interventions effective is critical to decisions about how this strategy should be applied to populations of heart failure patients. The data are mixed on whether greater frequency of monitoring leads to better outcomes. The findings by Benatar et al that daily physiological monitoring was more effective in reducing hospital readmissions compared with periodic home visits suggests that frequency of monitoring may be important. However, in the study by Cleland et al, monthly phone calls by nurses were essentially equivalent (in affecting outcomes) to twice-daily physiologic monitoring. While the interventions in the DIAL trial and the studies by Dunagan and Cleland included a component of active patient management, this does not seem to be a distinguishing feature between effective and ineffective interventions. While 3 out of the 5 telephone-based monitoring studies demonstrated a benefit, the negative studies by Riegel and DeBusk are notable. In Riegel’s study of Hispanic patients, the authors point out that nurses had difficulty reaching patients at various times during the follow-up period because they were moving among different households or traveling back to Mexico. Nurses therefore focused more on contacts with family, which may not be as potent as direct patient contact. The DeBusk study did specifically enroll patients deemed to be low risk for hospital readmission (on the basis of medical and sociodemographic characteristics), which may explain the negative findings. The authors also point out that their study participants (all enrolled in a Health Maintenance Organization) “all had access to high-quality health care, and they used it extensively.” The negative findings of Goldberg’s study of automated monitoring also require careful consideration. It is possible that the quality of usual care received by these patients who were all followed at heart failure specialty clinics left little room for improvement: a “ceiling effect” similar to the one seen in the study by DeBusk. Since many patients do not have access to heart failure specialty clinics, there remains a need to investigate other approaches that can be used in a variety of practice settings.
As in many areas in medicine, telemonitoring is in rapid evolution and appears to be moving towards more technically sophisticated and invasive forms of interventions. Recently completed but as yet unpublished trials are employing invasive monitoring of cardiac pressures with automated transmission of data (21). While preliminary results suggest this approach may have value in improving outcomes (22), such strategies will have to be evaluated for cost-effectiveness, scalability, safety, and acceptability to patients. Given the ever-growing number of patients affected by chronic diseases such as heart failure, the Centers for Medicare and Medicaid Services and other major payers cannot afford to invest in expensive telemonitoring interventions for which the incremental increase in cost (compared with less expensive interventions) is not proportional to an improvement in outcomes. Our review suggests that telephone-based systems of monitoring are less expensive and appear equally effective when compared with more complex forms of monitoring (15), though this needs to be confirmed in larger studies. While the Institute of Medicine’s report “Crossing the Quality Chasm” states that “[information technology] must play a central role in the redesign of the health care system,” to improve quality of care for patients with chronic disease such as heart failure (1), careful consideration of how best to implement such interventions is needed.
Figure 1.
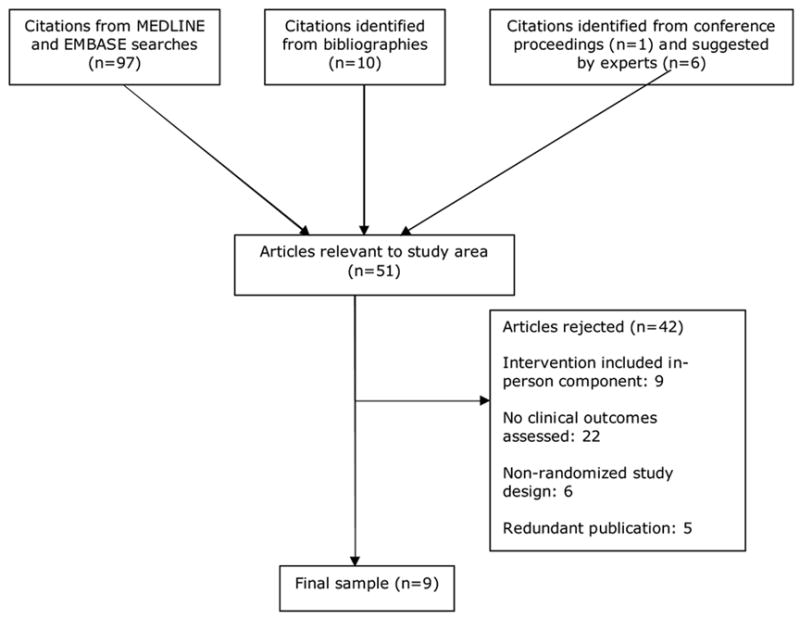
Literature search and selection process.
Table 1. Telephone-Based Symptom Monitoring.
| DIAL | Dunagan | Riegel | Riegel | DeBusk | ||
|---|---|---|---|---|---|---|
| Year published | 2005 | 2005 | 2002 | 2006 | 2004 | |
| Country | Argentina | USA | USA | USA | USA | |
| Study design/no. quality criteria met | RCT / 6 | RCT / 6 | RCT / 4 | RCT/6 | RCT / 6 | |
| Sample size/eligibility proportion | 1,518 / 0.64 | 151/0.20 | 358 / 0.31 | 134/0.32 | 462 / 0.30 | |
| Description of study population | Stable outpatients from 51 centers in Argentina | Hospitalized with HF | Hospitalized for HF | Hispanics hospitalized with HF | Recently hospitalized for HF and deemed low-risk for readmission | |
| Men (%) | 71 | 56 | 49 | 46 | 51 | |
| Mean age (SD) | 65 (13) | 70 (13) | 72 (12) | 72 (11) | 72 (11) | |
| HF severity | 50% NYHA class III/IV | 72% NYHA class III; 9% NYHA class IV | 38% NYHA class III; 59% class IV | 81% NYHA class III/IV | 49% NYHA class I/II; 51% class III/IV | |
| ACE/ARB at baseline (%) | 93 | 71 | 54 | 80 | 83 | |
| Beta-blocker at baseline (%) | 62 | NA | 17 | 54 | 35 | |
| Intervention: nature and intensity | Calls every 2 weeks for 8 weeks, then at a frequency determined by patient’s status over 12 month f/u period | Calls every week for 2 weeks, then at a frequency determined by patient’s status over 12 month f/u period | 17 calls over 6-month f/u period for a total of 16 hours of time | 14 calls to patients and 8 calls to families over 6-month f/u period | Weekly calls for 6 weeks with gradually decreasing frequency thereafter over 12 month f/u period | |
| Primary Outcome | HF hospitalization and all-cause mortality | All-cause hospitalization and emergency visits | HF hospitalization | HF hospitalization | Hospitalization for HF and all-cause | |
| All-cause hospitalization, event rate | RR (95% CI) | 261/760 I 296/758 C 0.85 (0.72–0.99) | 28/76 I 49/75 C 0.56 (0.40–0.79) | 81/130 I 198/228 C 0.72 (0.62–0.83) | 40/69 I 37/65 U 1.02 (0.76–1.36) | 116/228 I 117/234 C 0.98 (0.76–1.27) |
| HF hospitalization, event rate | RR (95% CI) | 128/760 I 169/758 C 0.71 (0.56–0.91) | 23/76 I 35/75 C 0.65 (0.43–0.99) | 23/130 I 63/228 C 0.64 (0.42–0.98) | 22/69 I 22/65 U 0.94 (0.58–1.53) | 76/228 I 86/234 C 0.91 (0.71–1.16) |
| Mortality, event rate | RR (95% CI) | 116/760 I 122/758 C 0.95 (0.73–1.23) | 6/76 I 5/75 C 1.17 (0.36–3.84) | 32/228 I 16/130 C 0.88 (0.50–1.54) | 5/69 I 8/65 U 0.59 (0.20–1.71) | 21/228 I 29/234 0.77 (0.45–1.32) |
Eligibility proportion refers to the number of patients screened for participation divided by the number actually enrolled in the study.ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CI, confidence interval; HF, heart failure; NA, not available; NYHA, New York Heart Association; RCT, randomized controlled trial; RR, relative risk; SD, standard deviation; I, intervention group; C, control group
Table 2. Automated Monitoring of Signs and Symptoms.
| Goldberg | |||
|---|---|---|---|
| Year published | 2003 | ||
| Country | USA | ||
| Study design/no. quality criteria met | RCT / 5 | ||
| Sample size/eligibility proportion | 280 / NA | ||
| Description of study population | Recently hospitalized for HF, recruited from HF centers | ||
| Men (%) | 68 | ||
| Mean age (SD) | 59 (15) | ||
| HF severity | 75% NYHA class III, 25% class IV | ||
| ACE/ARB at baseline (%) | 74 | ||
| Beta-blocker at baseline (%) | 38 | ||
| Intervention: nature and intensity | Twice daily monitoring of symptoms and weight for 6 months | ||
| Primary outcome | All-cause hospitalization | ||
| All-cause hospitalizations, event rate | RR (95% CI) | NA | 0.85 (0.6–1.25) |
| HF hospitalizations, event rate | RR (95% CI) | NA | NA |
| Mortality, event rate | RR (95% CI) | 11/138 I 26/142 C | 0.44 (0.22–0.85) |
Eligibility proportion refers to the number of patients screened for participation divided by the number actually enrolled in the study.ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CI, confidence interval; HF, heart failure; NA, not available; NYHA, New York Heart Association; RCT, randomized controlled trial; RR, relative risk; SD, standard deviation; I, intervention group; C, control group
Table 3. Automated Physiologic Monitoring.
| Benatar | |||
|---|---|---|---|
| Year published | 2003 | ||
| Country | USA | ||
| Study design/no. quality criteria met | Randomized trial / 2 | ||
| Sample size/eligibility proportion | 216 / 0.79 | ||
| Description of study population | Outpatients from 2 academic centers | ||
| Men (%) | 37 | ||
| Mean age (SD) | 63 (12) | ||
| HF severity | Mean NYHA class 3.1 (SD 0.26) | ||
| ACE/ARB at baseline (%) | 75 | ||
| Beta-blocker at baseline (%) | 53 | ||
| Intervention: nature and intensity | For 3 months, daily monitoring of weight, blood pressure, heart rate and oxygen saturation compared with home nurse visits | ||
| Primary outcome | Not specified | ||
| All-cause hospitalizations, absolute event rate | RR (95% CI) | NA | NA |
| HF hospitalizations, event rate | RR (95% CI) | 38/108 DM 63/108 HNV | 0.60 (0.45–0.82) |
| Mortality, event rate | RR (95% CI) | NA | NA |
Eligibility proportion refers to the number of patients screened for participation divided by the number actually enrolled in the study.
ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CI, confidence interval; HF, heart failure; NA, not available; NYHA, New York Heart Association; RCT, randomized controlled trial; RR, relative risk; SD, standard deviation; DM, daily monitoring group; HNV, home nurse visits
Table 4. Comparison of ≥2 Methods of Telemonitoring.
| Cleland | Jerant | ||||
|---|---|---|---|---|---|
| Year published | 2005 | 2001 | |||
| Country | 3 European countries (Germany, the Netherlands, UK) | USA | |||
| Study design/no. quality criteria met | RCT / 6 | RCT / 4 | |||
| Sample size/eligibility proportion | 426 / NA | 37 / 0.11 | |||
| Description of study population | Recently hospitalized for HF | Recently hospitalized for HF | |||
| Men (%) | 78 | 46 | |||
| Mean age (SD) | 67 (11) | 70 (12) | |||
| HF severity | 62% NYHA class I/II | 65% NYHA class II, 33% class III | |||
| ACE/ARB at baseline (%) | 81 | 65 | |||
| Beta-blocker at baseline (%) | 51 | 38 | |||
| Intervention: nature and intensity | Over 240 days, patients randomized to: usual care (UC); physiologic monitoring consisting of twice-daily self-measurement of weight, blood pressure, heart rate, and rhythm (HTM); and monthly phone calls by an HF specialist nurse to assess symptoms and medication (NTS) | Interventions provided over 60 days with frequency determined by patients’ status. Patients randomized to receive: video conferencing with an integrated stethoscope, nursing support by telephone and usual care | |||
| Primary Outcome | Days lost to death or all-cause hospitalization | HF hospitalization charges | |||
| All-cause hospitalizations, event rate | RR (95% CI) | 85/173 NTS | NTS vs UC 0.91 (0.71–1.16) | 9/13 Video conferencing | Unable to calculate due to small numbers |
| 80/168 HTM | HTM vs UC 0.88 (0.68–1.13) | 5/12 Nursing support | |||
| 46/85 UC | HTM vs NTS 0.97 (0.78–1.21) | 15/12 Usual care | |||
| HF hospitalizations, event rate | RR (95% CI) | 34/173 NTS | NTS vs UC 0.70 (0.44–1.10) | 1/13 Video conferencing | Unable to calculate due to small numbers |
| 40/168 HTM | HTM vs UC 0.84 (0.55–1.30) | 1 /12 Nursing support | |||
| 24/85 UC | HTM vs NTS 1.21 (0.81–1.82) | 4 /12 Usual care | |||
| Mortality, event rate | RR (95% CI) | 27/173 NTS | NTS vs UC 0.66 (0.40–1.11) | 0/13 Video conferencing | Unable to calculate due to small numbers |
| 28/168 HTM | HTM vs UC 0.71 (0.42–1.18) | 2/12 Nursing support | |||
| 20/85 UC | HTM vs NTS 1.07 (0.66–1.73) | 0/12 Usual care |
Eligibility proportion refers to the number of patients screened for participation divided by the number actually enrolled in the study.
ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CI, confidence interval; HF, heart failure; HTM, home telemonitoring; NA, not available; NTS, nurse telephone support; NYHA, New York Heart Association; RCT, randomized controlled trial; RR, relative risk; SD, standard deviation; UC, usual care
Footnotes
Dr. Phillips is supported by Supplement 3 R01 HL080228-01S1 from the National Heart, Lung, and Blood Institute.
Dr. Stewart is supported by the NHF and NHMRC of Australia.
Disclosures: “Drs. Phillips’, Chaudhry’s and Krumholz’s support from NHLBI, and Dr. Stewart’s support from the NHF and NHMRC of Australia had no role in the conduct of this study. Drs. Jerant and Riegel were investigators in studies discussed in this paper (15,16). Drs. Chaudhry and Krumholz are investigators in an ongoing trial of telemonitoring (funded by NHLBI) in heart failure patients. Dr. Krumholz is a member of the advisory board for Alere Medical Inc®.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute of Medicine (U.S.) Crossing the quality chasm : a new health system for the 21st century. Washington, D.C.: National Academy Press; 2001. Committee on Quality of Health Care in America. [PubMed] [Google Scholar]
- 2.Chumbler NR, Vogel WB, Garel M, Qin H, Kobb R, Ryan P. Health services utilization of a care coordination/home-telehealth program for veterans with diabetes: a matched-cohort study. J Ambul Care Manage. 2005;28:230–40. doi: 10.1097/00004479-200507000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Cavallerano AA, Cavallerano JD, Katalinic P, et al. A telemedicine program for diabetic retinopathy in a Veterans Affairs Medical Center--the Joslin Vision Network Eye Health Care Model. Am J Ophthalmol. 2005;139:597–604. doi: 10.1016/j.ajo.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro SE, Izumi S, Tanner CA, et al. Telephone advice nursing services in a US health maintenance organization. J Telemed Telecare. 2004;10:50–4. doi: 10.1258/135763304322764202. [DOI] [PubMed] [Google Scholar]
- 5.Super N. National Health Policy Forum. 2004. Medicare’s Chronic Care Improvement Pilot Program: What Is Its Potential? [PubMed] [Google Scholar]
- 6.Kleinpell RM, Avitall B. Telemanagement in Chronic Heart Failure: A Review. Disease Management Health Outcomes. 2005;13:43–52. [Google Scholar]
- 7.Louis AA, Turner T, Gretton M, Baksh A, Cleland JG. A systematic review of telemonitoring for the management of heart failure. Eur J Heart Fail. 2003;5:583–90. doi: 10.1016/s1388-9842(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–64. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 9.DeBusk RF, Miller NH, Parker KM, et al. Care management for low-risk patients with heart failure: a randomized, controlled trial. Ann Intern Med. 2004;141:606–13. doi: 10.7326/0003-4819-141-8-200410190-00008. [DOI] [PubMed] [Google Scholar]
- 10.Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ. 2005;331:425. doi: 10.1136/bmj.38516.398067.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine. Telemedicine: A Guide to Assessing Telecommunications in Health Care. Washington DC: National Academy Press; 1996. [PubMed] [Google Scholar]
- 12.Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91:899–906. doi: 10.1136/hrt.2004.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juni P, Altman D, Egger M. Systematic Reviews in Health Care: Meta-Analysis in Context. London: BMJ Publishing Group; 2001. Assessing the Quality of Randomised Controlled Trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centre for Reviews and Dissemination. Undertaking Systematic Review of Research on Effectiveness. York: University of York; 2001. [Google Scholar]
- 15.Jerant AF, Azari R, Nesbitt TS. Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention. Medical Care. 2001;39:1234–45. doi: 10.1097/00005650-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Archives of Internal Medicine. 2002;162:705–12. doi: 10.1001/archinte.162.6.705. [DOI] [PubMed] [Google Scholar]
- 17.Dunagan WC, Littenberg B, Ewald GA, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Card Fail. 2005;11:358–65. doi: 10.1016/j.cardfail.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Card Fail. 2006;12:211–9. doi: 10.1016/j.cardfail.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg LR, Piette JD, Walsh MN, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. American Heart Journal. 2003;146:705–12. doi: 10.1016/S0002-8703(03)00393-4. [DOI] [PubMed] [Google Scholar]
- 20.Benatar D, Bondmass M, Ghitelman J, Avitall B. Outcomes of chronic heart failure. Archives of Internal Medicine. 2003;163:347–52. doi: 10.1001/archinte.163.3.347. [DOI] [PubMed] [Google Scholar]
- 21.Kjellstrom B, Igel D, Abraham J, Bennett T, Bourge R. Trans-telephonic monitoring of continuous haemodynamic measurements in heart failure patients. J Telemed Telecare. 2005;11:240–4. doi: 10.1258/1357633054471795. [DOI] [PubMed] [Google Scholar]
- 22. [Last accessed March 6, 2006.]; http://wwwp.medtronic.com/Newsroom/NewsReleaseDetails.do?itemId=1110237750252&lang=en_US.


