Abstract
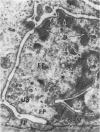
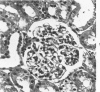
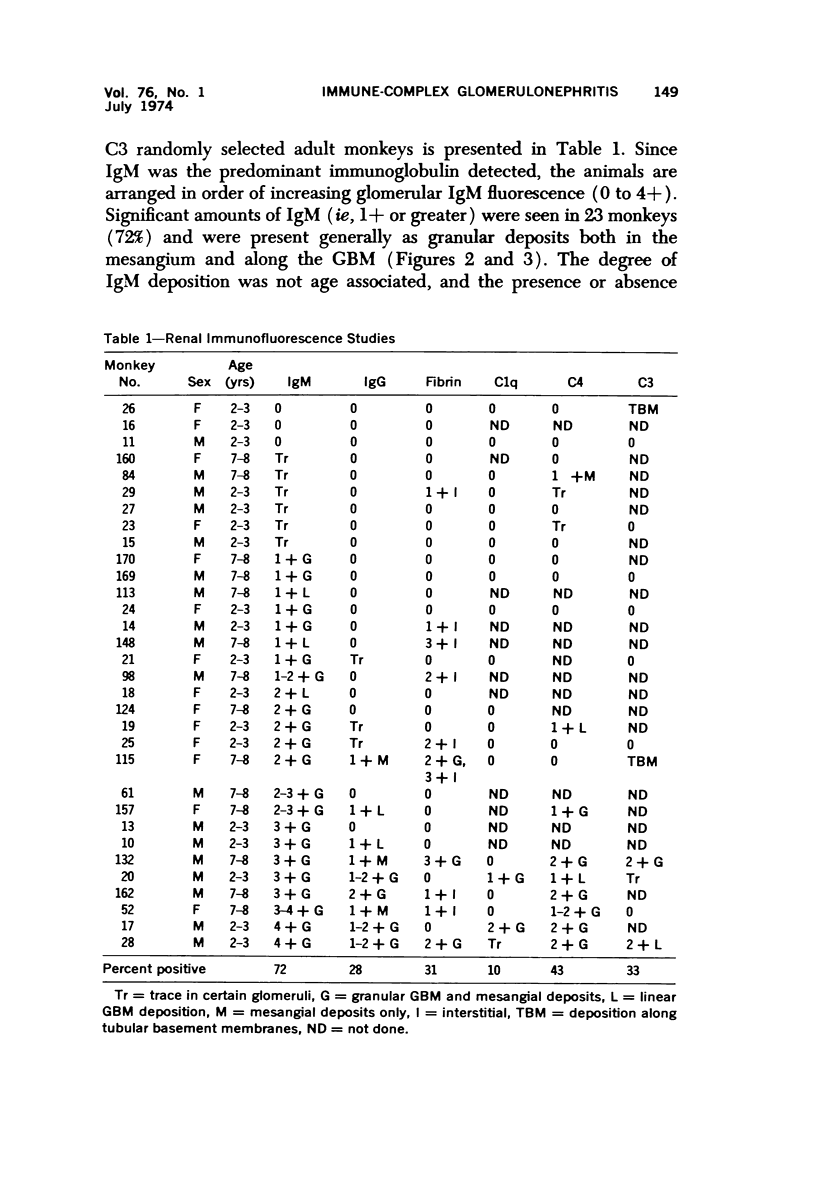
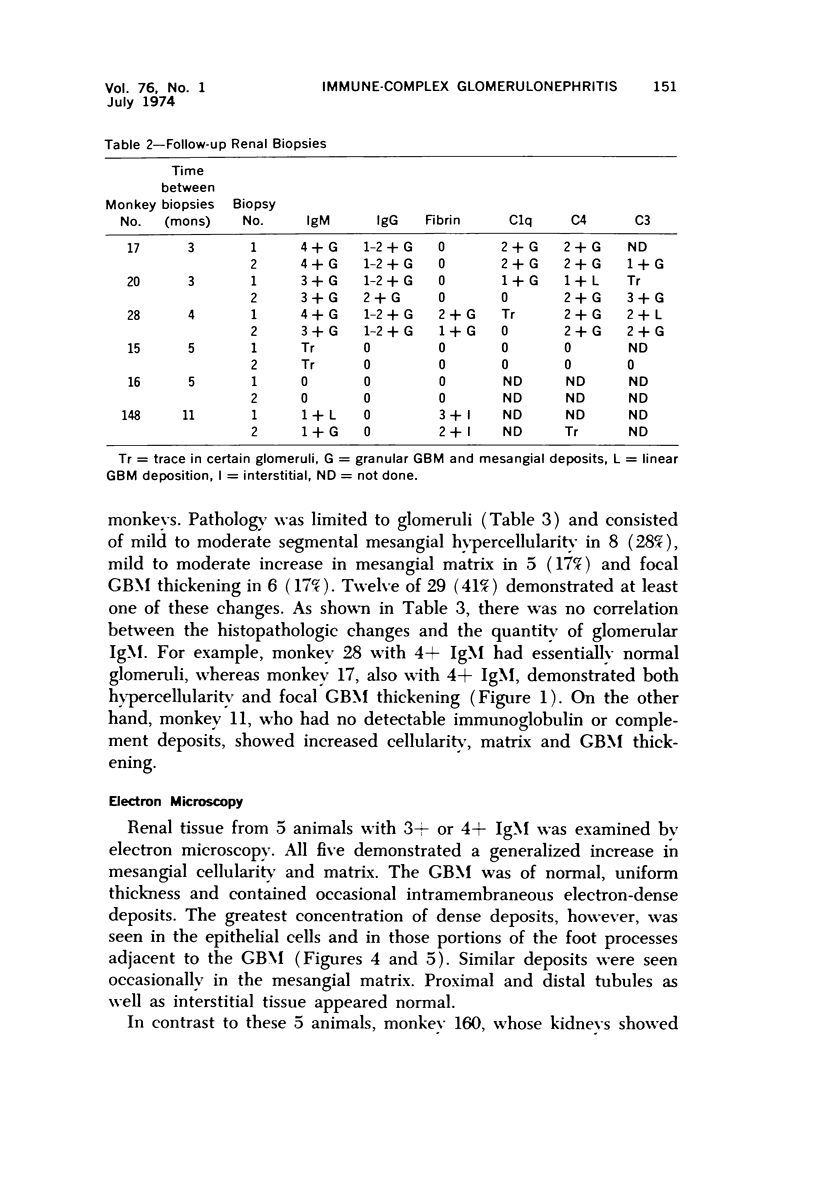
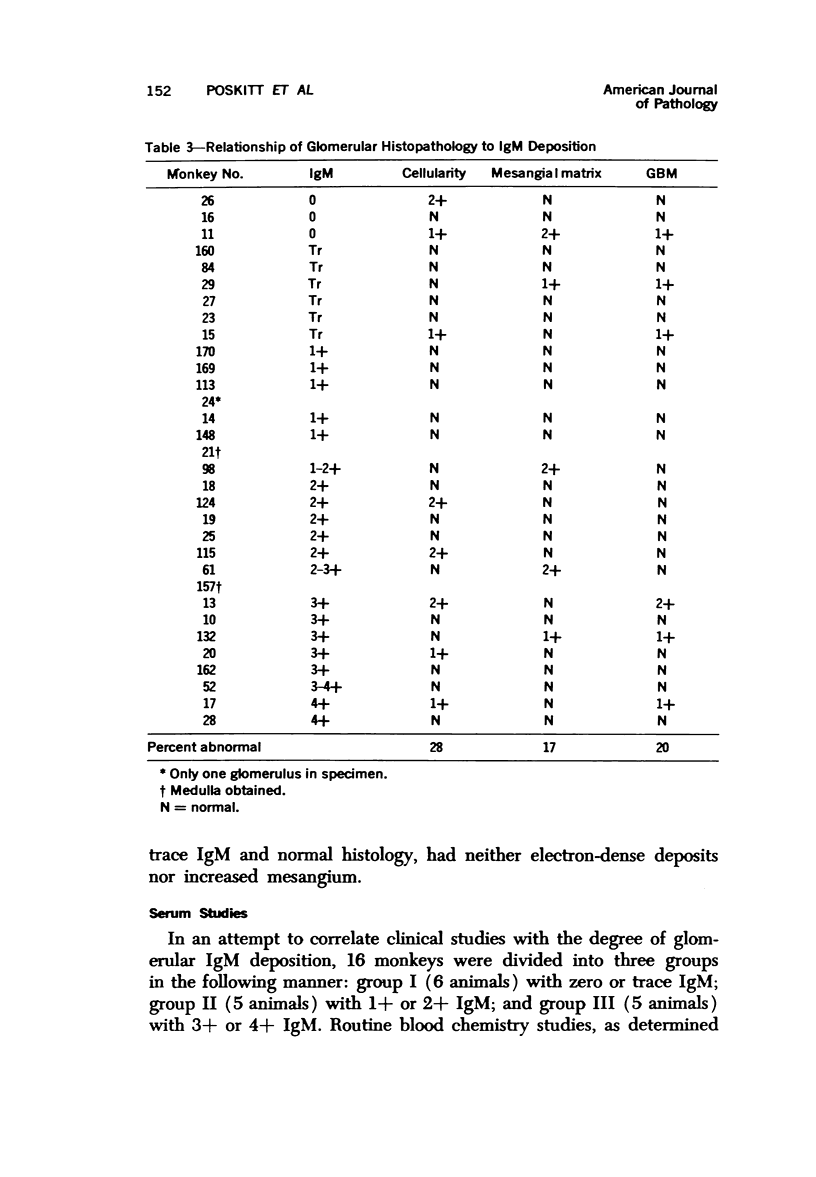
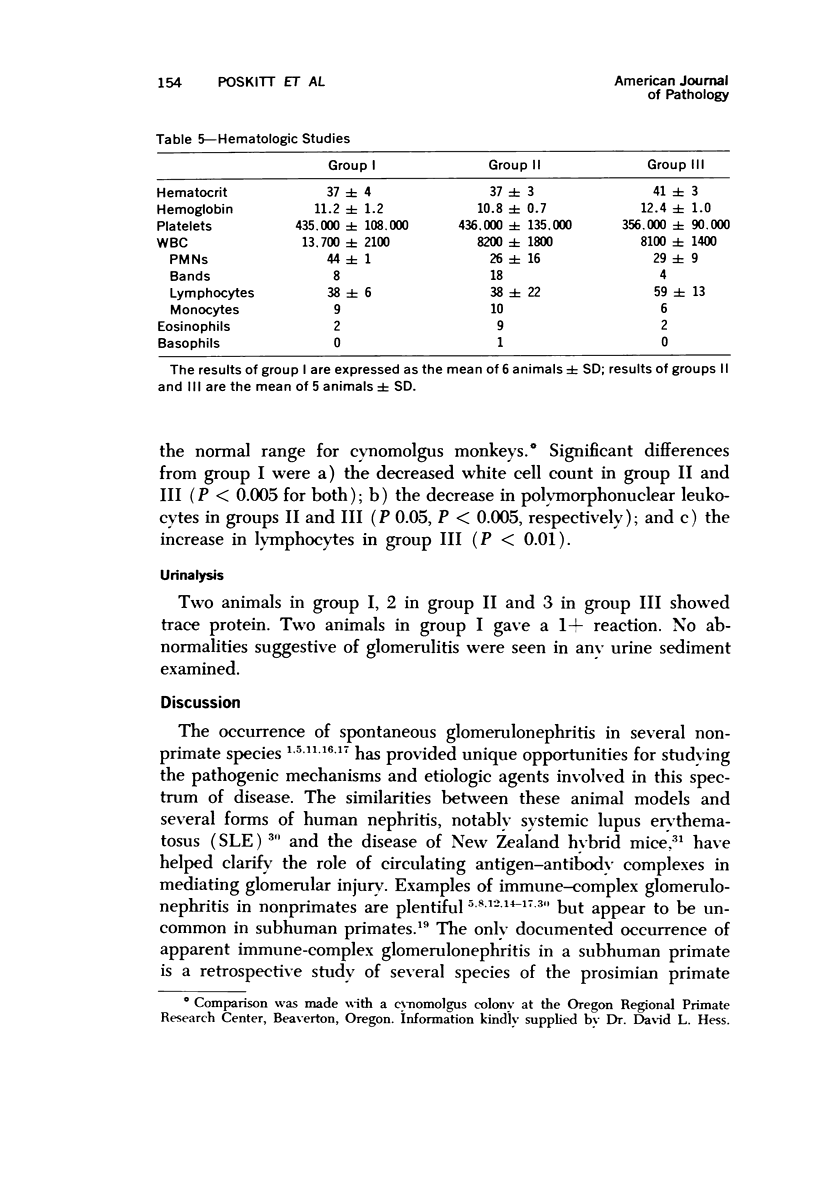
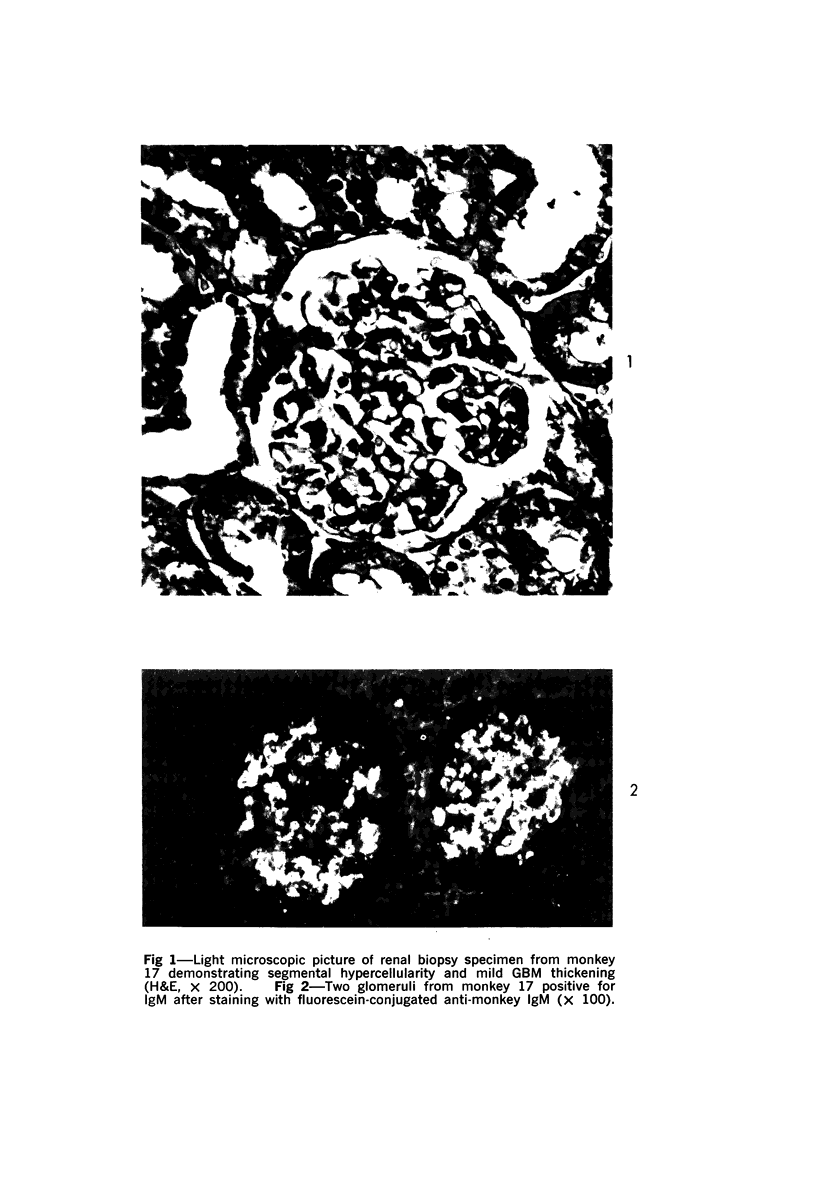
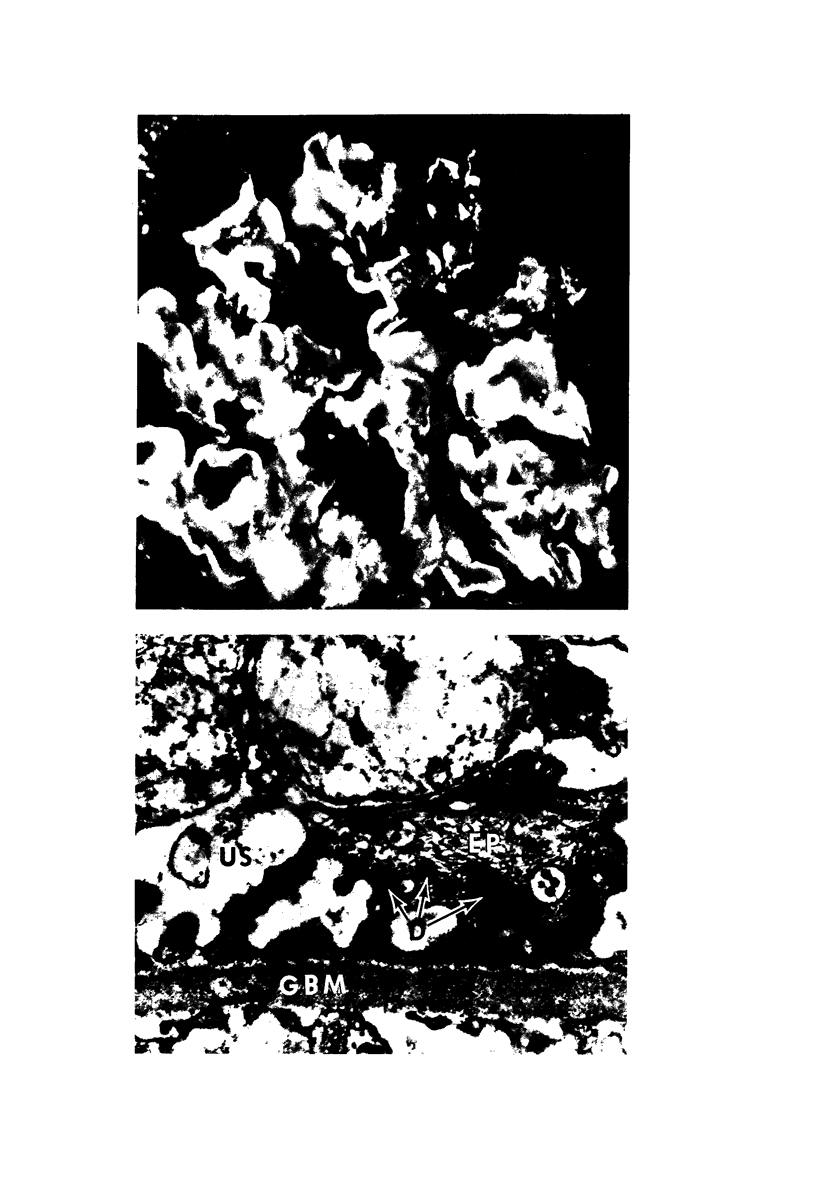
Light, immunofluorescence and electron microscopic studies were carried out on renal biopsies from 32 randomly selected adult monkeys (Macaca irus). Histopathology was limited to glomeruli and consisted of mild to moderate segmental increases in mesangial cells, mesangial matrix, and/or glomerular basement membrane (GBM) thickness in 41% of the animals. Granular deposits of IgM were present in the mesangial region and along the GBM in 72% of the monkeys, whereas IgG, C1q, C4 and C3 were detected in approximately 30%. Electrondense deposits were seen predominantly in epithelial foot processes adjacent to the GBM and, to a lesser extent, in the mesangium. Those monkeys with the heaviest IgM deposition were found to have decreased serum levels of C3, IgM and IgA. Follow-up biopsies over a period of 3 to 11 months revealed that the disease process was persistent yet nonprogressive. No correlation with age or sex was noted. All animals examined were clinically healthy and had normal renal function. This is the first documented occurrence of spontaneous immune-complex glomerulonephritis in a large monkey population. It appears to be a persistent disease which does not progress to renal insufficiency and which may serve as an investigative model for mild nonprogressive forms of human glomerulonephritis.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altshuler H. L., Stowell R. E., Lowe R. T. Normal serum biochemical values of Macaca arctoides, Macaca fascicularis, and Macaca radiata. Lab Anim Sci. 1971 Dec;21(6):916–926. [PubMed] [Google Scholar]
- Anderson L. J., Jarrett W. F. Membranous glomerulonephritis associated with leukaemia in cats. Res Vet Sci. 1971 Mar;12(2):179–180. [PubMed] [Google Scholar]
- Banks K. L., Henson J. B., McGuire T. C. Immunologically mediated glomerulitis of horses. I. Pathogenesis in persistent infection by equine infectious anemia virus. Lab Invest. 1972 Jun;26(6):701–707. [PubMed] [Google Scholar]
- Burkholder P. M., Bergeron J. A. Spontaneous glomerulonephritis in the prosimian primate Galago. A correlative light, immunofluorescence and electron microscopic analysis. Am J Pathol. 1970 Dec;61(3):437–456. [PMC free article] [PubMed] [Google Scholar]
- CHAPLIN H., COHEN S., PRESS E. M. PREPARATION AND PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL HUMAN 19 S GAMMA-GLOBULIN (IGM). Biochem J. 1965 Apr;95:256–261. doi: 10.1042/bj0950256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Cheville N. F., Mengeling W. L., Zinober M. R. Ultrastructural and immunofluorescent studies of glomerulonephritis in chronic hog cholera. Lab Invest. 1970 May;22(5):458–467. [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune-complex pathogenesis of experimental allergic glomerulonephritis. Science. 1967 Mar 17;155(3768):1432–1434. doi: 10.1126/science.155.3768.1432. [DOI] [PubMed] [Google Scholar]
- Feltkamp T. E., Boode J. H. Elution of antibodies from biopsy tissue. J Clin Pathol. 1970 Oct;23(7):629–631. doi: 10.1136/jcp.23.7.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell R. E., Blakemore W. F. A case of immune complex glomerulonephritis in a dog. Vet Rec. 1972 Mar 4;90(10):275–280. doi: 10.1136/vr.90.10.275. [DOI] [PubMed] [Google Scholar]
- Kaur J., Chakravarti R. N., Chugh K. S., Chhuttani P. N. Spontaneously occuring renal diseases in wild rhesus monkeys. J Pathol Bacteriol. 1968 Jan;95(1):31–36. doi: 10.1002/path.1700950105. [DOI] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn K., Mero M., Oksanen A., Sandholm M. Immunologic observations in canine interstitial nephritis. Am J Pathol. 1971 Oct;65(1):157–172. [PMC free article] [PubMed] [Google Scholar]
- Kurtz J. M., Russell S. W., Lee J. C., Slauson D. O., Schechter R. D. Naturally occurring canine glomerulonephritis. Am J Pathol. 1972 Jun;67(3):471–482. [PMC free article] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Dixon F. J., Lee S. Spontaneous glomerulonephritis in sheep. II. Studies on natural history, occurrence in other species, and pathogenesis. Am J Pathol. 1968 Oct;53(4):501–512. [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Dixon F. J. Spontaneous glomerulonephritis in sheep. Lab Invest. 1966 Jul;15(7):1279–1289. [PubMed] [Google Scholar]
- Lewis R. M. Animal model: canine systemic lupus erythematosus. Am J Pathol. 1972 Dec;69(3):537–540. [PMC free article] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. Isolation of a thermolabile serum protein which precipitates gamma-globulin aggregates and participates in immune hemolysis. Proc Soc Exp Biol Med. 1961 Feb;106:291–295. doi: 10.3181/00379727-106-26313. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McCluskey R. T. The value of immunofluorescence in the study of human renal disease. J Exp Med. 1971 Sep 1;134(3 Pt 2):242s–255s. [PubMed] [Google Scholar]
- Mellors R. C., Shirai T., Aoki T., Huebner R. J., Krawczynski K. Wild-type Gross leukemia virus and the pathogenesis of the glomerulonephritis of New Zealand mice. J Exp Med. 1971 Jan 1;133(1):113–132. doi: 10.1084/jem.133.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M., Franklin E. C. Cryoglobulinemia--a study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med. 1966 Jun;40(6):828–836. doi: 10.1016/0002-9343(66)90199-9. [DOI] [PubMed] [Google Scholar]
- Meltzer M., Franklin E. C., Elias K., McCluskey R. T., Cooper N. Cryoglobulinemia--a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966 Jun;40(6):837–856. doi: 10.1016/0002-9343(66)90200-2. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lymphocytic choriomeningitis: production of antibody by "tolerant" infected mice. Science. 1967 Dec 1;158(3805):1193–1195. doi: 10.1126/science.158.3805.1193. [DOI] [PubMed] [Google Scholar]
- Pan I. C., Tsai K. S., Karstad L. Glomerulonephritis in Aleutian disease of mink: histological and immunofluorescence studies. J Pathol. 1970 Jun;101(2):119–127. doi: 10.1002/path.1711010207. [DOI] [PubMed] [Google Scholar]
- Poskitt T. R. Immunologic and electron microscopic studies in Goodpasture's syndrome. Am J Med. 1970 Aug;49(2):250–257. doi: 10.1016/s0002-9343(70)80081-x. [DOI] [PubMed] [Google Scholar]
- Slauson D. O., Russell S. W., Schechter R. D. Naturally occurring immune-complex glomerulonephritis in the cat. J Pathol. 1971 Feb;103(2):131–133. doi: 10.1002/path.1711030208. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Lai C. H., Chen S. T., Schaeffer B. T. Evolution of chronic glomerulonephritis induced in mice by ECHO-9 and Coxsackie B 1 viruses. J Pathol. 1971 May;104(1):53–57. doi: 10.1002/path.1711040107. [DOI] [PubMed] [Google Scholar]
- Weigle W. O., Nakamura R. M. Perpetuation of autoimmune thyroiditis and production of secondary renal lesions following periodic injections of aqueous preparations of altered thyroglobulin. Clin Exp Immunol. 1969 Jun;4(6):645–657. [PMC free article] [PubMed] [Google Scholar]