Abstract
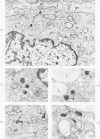
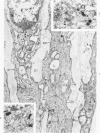
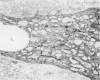
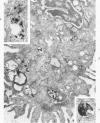
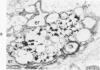
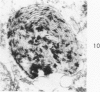
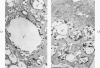
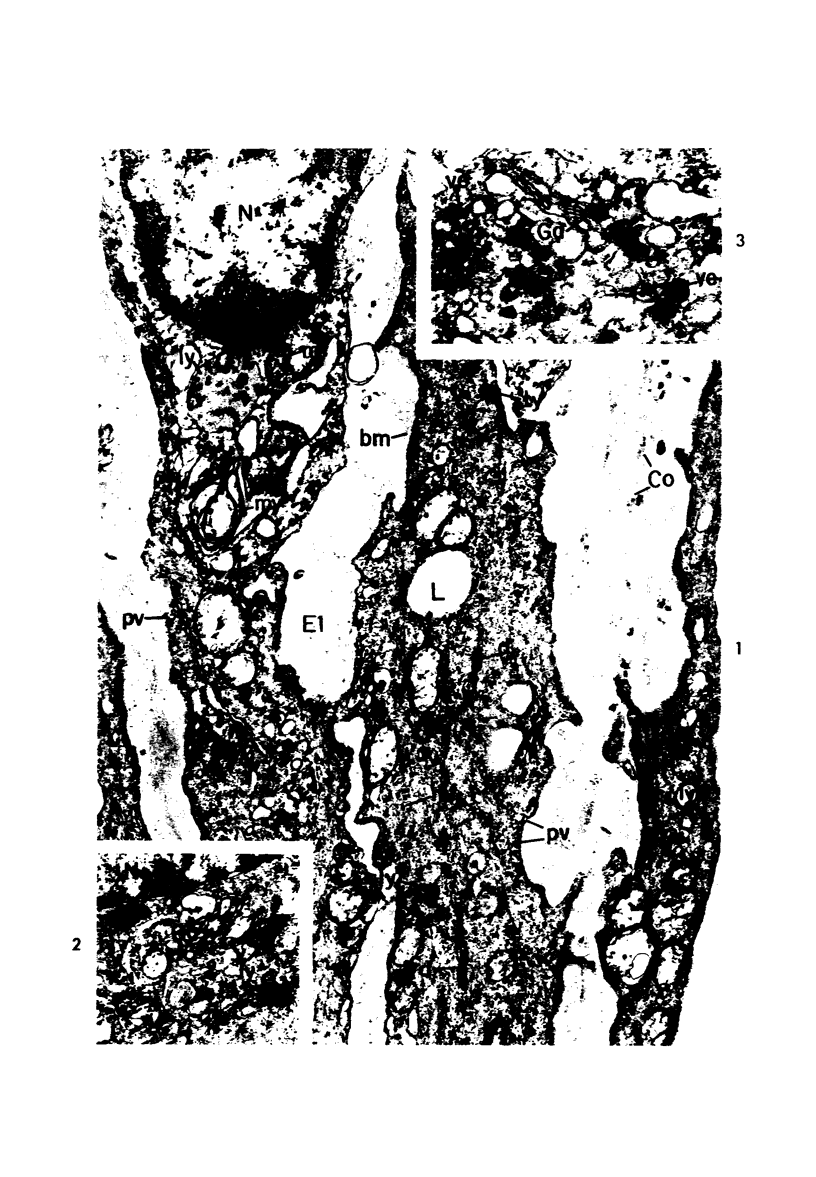
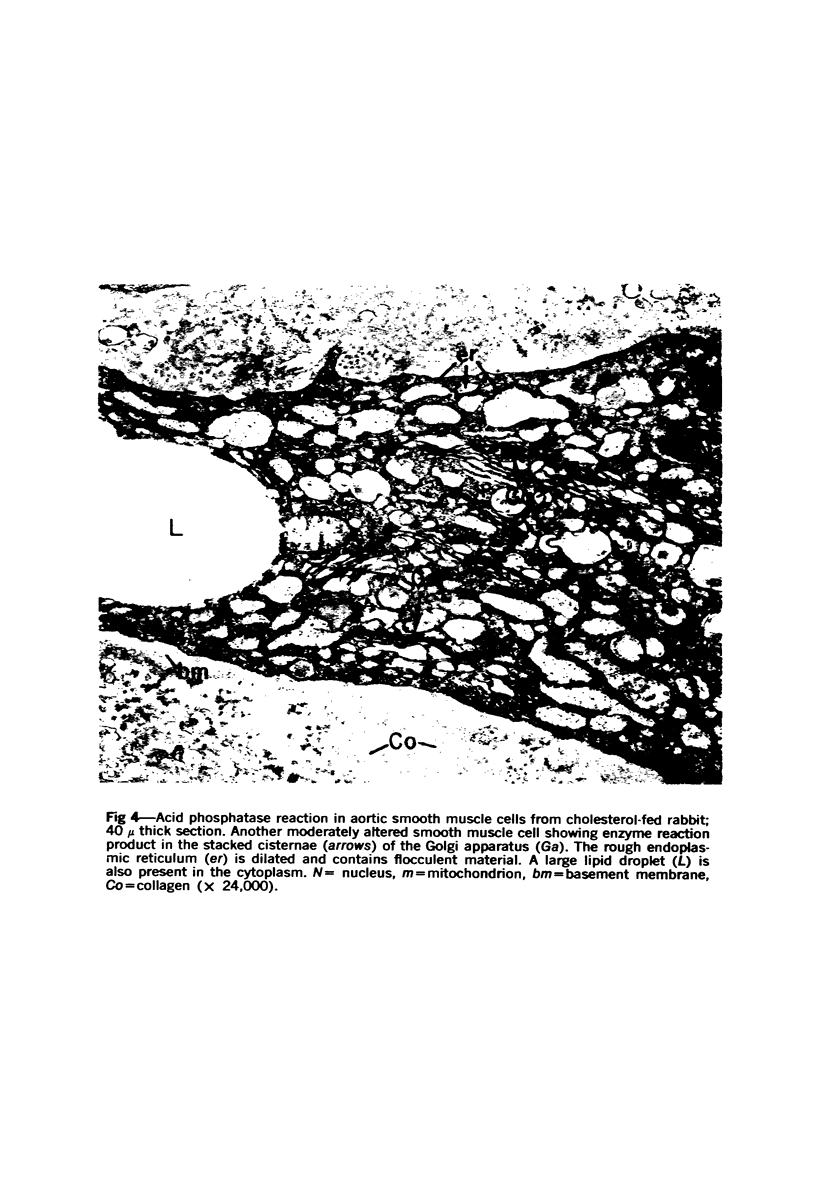
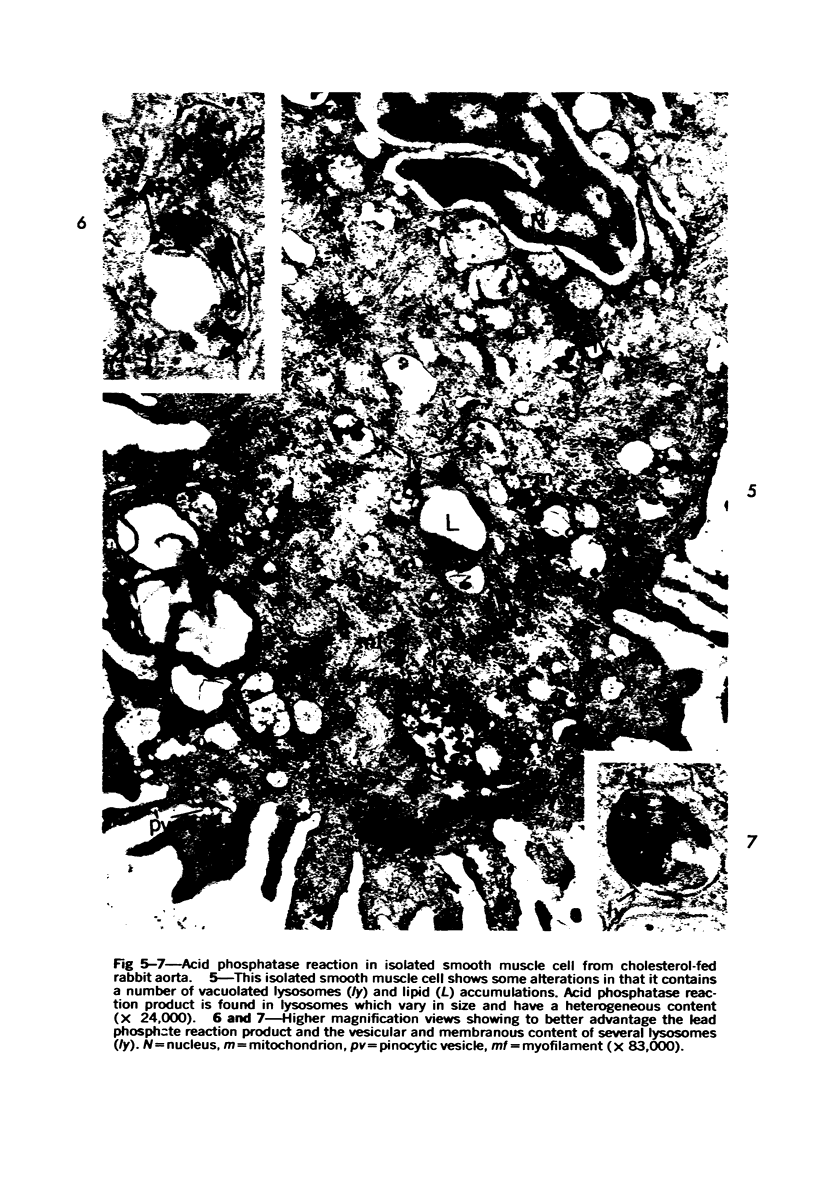
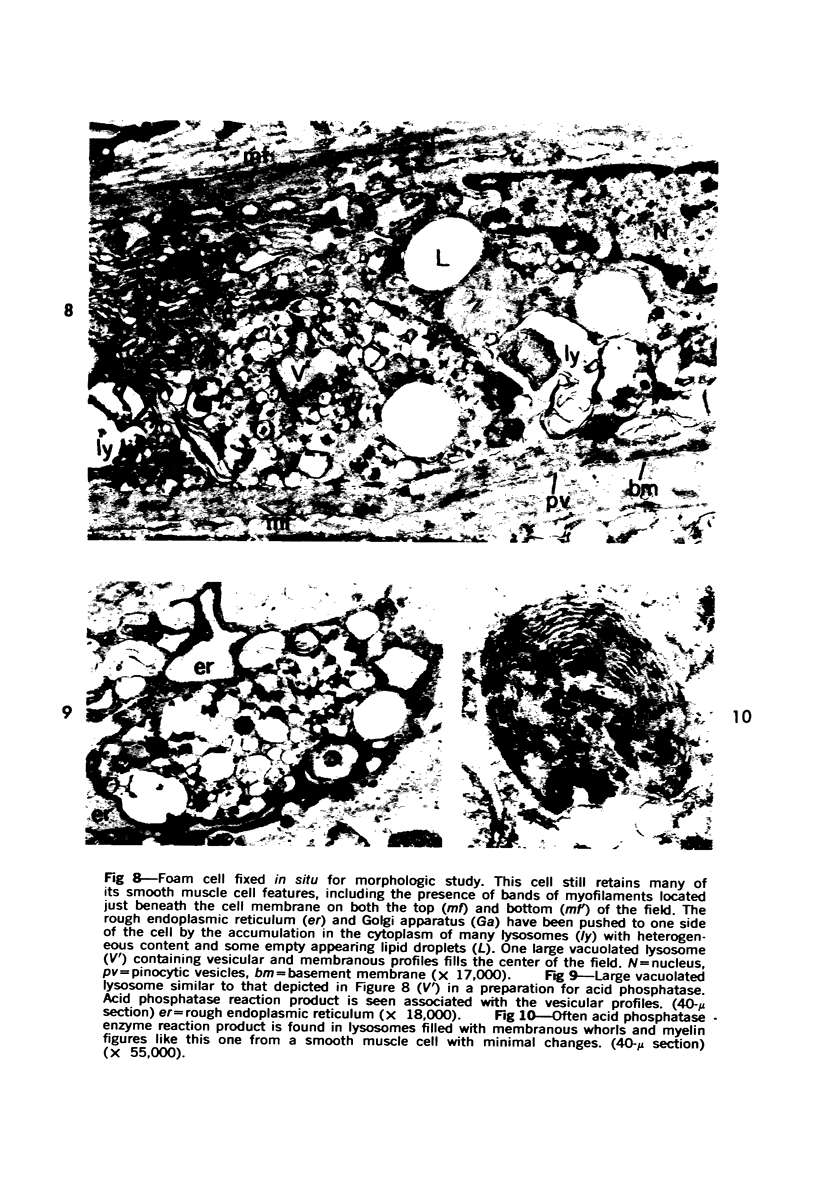
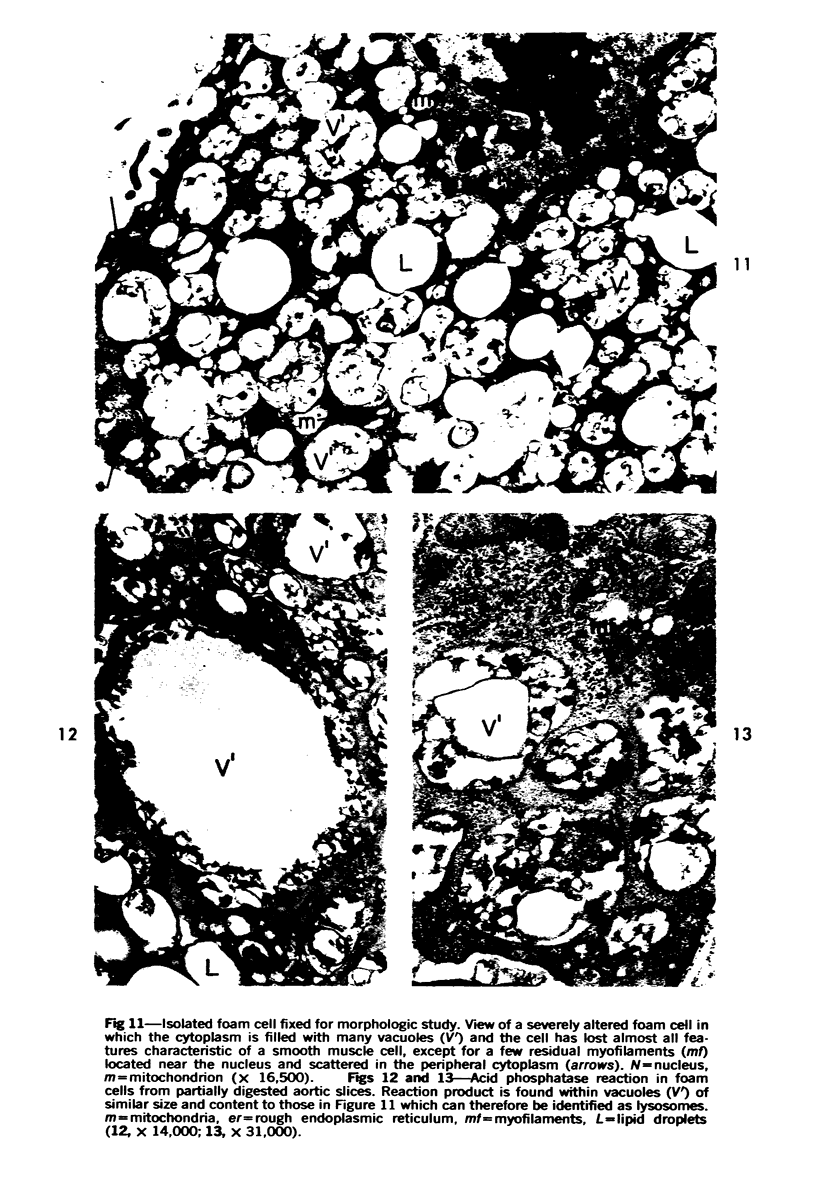
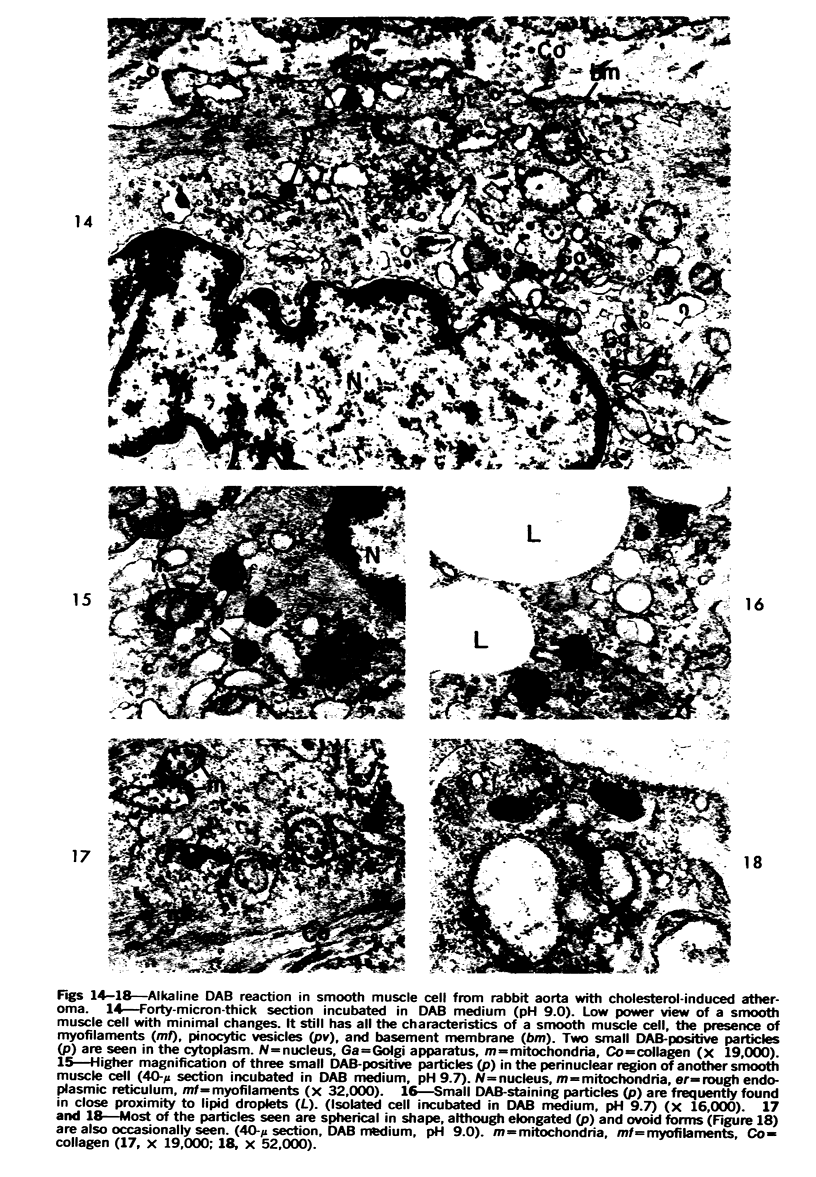
Cytochemical methods for acid phosphatase and catalase were applied to atheromatous aortas from cholesterol-fed rabbits. Whole tissue, partially digested aortic slices and isolated cells were used for the study. Present in the atheromatous lesions were smooth muscle cells in all stages of foamy transformation, from virtually normal appearing smooth muscle cells to severely altered cells with pronounced lipid accumulation. The results with the acid phosphatase method show that lysosomes increase both in size and in number as the smooth muscle cells become foam cells. In normal appearing smooth muscle cells, acid phosphatase reaction product was found in stacked cisternae of the Golgi apparatus and in small vesicles located in the Golgi region and distributed throughout the cytoplasm. In foam cells, reaction product was found in membrane-limited vacuoles of varying size which typically contained membranous debris or myelin-like figures together with massive lipid deposits. No reaction was seen in “free” cytoplasmic lipid droplets lacking a surrounding membrane. These results confirm and extend previous biochemical findings indicating that, in the cholesterol-fed rabbit, the change from normal smooth muscle cell to foam cell is accompanied by marked physical and chemical changes of the lysosomes, including their progressive overloading with cholesteryl ester. Small diaminobenzidine-positive particles were present in normal smooth muscle cells and in those at all stages of foamy transformation. These particles were more frequent in foam cells, in agreement with the marked increase in catalase activity detected biochemically in these cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCK R. C. The fine structure of the aortic endothelial lesions in experimental cholesterol atherosclerosis of rabbits. Am J Pathol. 1958 Sep-Oct;34(5):897–909. [PMC free article] [PubMed] [Google Scholar]
- Barbolini G., Scilabra G. A., Botticelli A., Botticelli S. On the origin of foam cells in cholesterol-induced atherosclerosis of the rabbit. Virchows Arch B Cell Pathol. 1969;3(1):24–32. doi: 10.1007/BF02901924. [DOI] [PubMed] [Google Scholar]
- Caravaca J., Dimond E. G., Sommers S. C., Wenk R. Prevention of induced atherosclerosis by peroxidase. Science. 1967 Mar 10;155(3767):1284–1287. doi: 10.1126/science.155.3767.1284. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEER J. C., McGILL H. C., Jr, STRONG J. P. The fine structure of human atherosclerotic lesions. Am J Pathol. 1961 Mar;38:263–287. [PMC free article] [PubMed] [Google Scholar]
- Gupta P. P., Tandon H. D., Ramalingaswami V. Experimental atherosclerosis in rabbits with special reference to reversal. J Pathol. 1970 Aug;101(4):309–317. doi: 10.1002/path.1711010403. [DOI] [PubMed] [Google Scholar]
- Hruban Z., Vigil E. L., Slesers A., Hopkins E. Microbodies: constituent organelles of animal cells. Lab Invest. 1972 Aug;27(2):184–191. [PubMed] [Google Scholar]
- Imai H., Lee K. T., Pastori S., Panlilio E., Florentin R., Thomas W. A. Atherosclerosis in rabbits. Architectural and subcellular alterations of smooth muscle cells of aortas in response to hyperlipemia. Exp Mol Pathol. 1966 Jun;5(3):273–310. doi: 10.1016/0014-4800(66)90036-0. [DOI] [PubMed] [Google Scholar]
- KEECH M. K. Electron microscope study of the normal rat aorta. J Biophys Biochem Cytol. 1960 Jun;7:533–538. doi: 10.1083/jcb.7.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOJDA Z. The enzyme topochemistry of the arterial wall. Cesk Morfol. 1962;10:46–61. [PubMed] [Google Scholar]
- Marshall J. R., Adams J. G., O'Neal R. M., De Bakey M. E. The ultrastructure of uncomplicated human atheroma in surgically resected aortas. J Atheroscler Res. 1966 Mar-Apr;6(2):120–131. doi: 10.1016/s0368-1319(66)80017-0. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Goldfischer S. Visualization of peroxisomes (microbodies) and mitochondria with diaminobenzidine. J Histochem Cytochem. 1969 Oct;17(10):675–680. doi: 10.1177/17.10.675. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. II. A cytochemical method for light and electron microscopy. J Histochem Cytochem. 1972 Dec;20(12):1006–1023. doi: 10.1177/20.12.1006. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. V. Are microperoxisomes ubiquitous in mammalian cells? J Histochem Cytochem. 1973 Aug;21(8):737–755. doi: 10.1177/21.8.737. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Microperoxisomes. J Histochem Cytochem. 1973 Nov;21(11):963–966. doi: 10.1177/21.11.963. [DOI] [PubMed] [Google Scholar]
- PEASE D. C., PAULE W. J. Electron microscopy of elastic arteries; the thoracic aorta of the rat. J Ultrastruct Res. 1960 Jun;3:469–483. doi: 10.1016/s0022-5320(60)90023-x. [DOI] [PubMed] [Google Scholar]
- Parker F., Odland G. F. A correlative histochemical, biochemical and electron microscopic study of experimental atherosclerosis in the rabbit aorta with special reference to the myo-intimal cell. Am J Pathol. 1966 Feb;48(2):197–239. [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., De Duve C. Lysosomes of the arterial wall. II. Subcellular fractionation of aortic cells from rabbits with experimantal atheroma. Exp Mol Pathol. 1974 Apr;20(2):228–256. doi: 10.1016/0014-4800(74)90057-4. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K. Possible properties of microbodies (peroxisomes). Microbody proliferation and hypolipidemic drugs. J Histochem Cytochem. 1973 Nov;21(11):967–971. doi: 10.1177/21.11.967. [DOI] [PubMed] [Google Scholar]
- Reddy J., Svoboda D. Microbodies (peroxisomes) in the interstitial cells of rodent testes. Lab Invest. 1972 Jun;26(6):657–665. [PubMed] [Google Scholar]
- STILL W. J. AN ELECTRON MICROSCOPE STUDY OF CHOLESTEROL ATHEROSCLEROSIS IN THE RABBIT. Exp Mol Pathol. 1963 Dec;14:491–502. doi: 10.1016/0014-4800(63)90026-1. [DOI] [PubMed] [Google Scholar]
- STILL W. J., MARRIOTT P. R. COMPARATIVE MORPHOLOGY OF THE EARLY ATHEROSCLEROTIC LESION IN MAN AND CHOLESTEROL-ATHEROSCLEROSIS IN THE RABBIT AN ELECTRONMICROSCOPIC STUDY. J Atheroscler Res. 1964 Sep-Oct;4:373–386. doi: 10.1016/s0368-1319(64)80023-5. [DOI] [PubMed] [Google Scholar]
- Smith E. B., Evans P. H., Downham M. D. Lipid in the aortic intima. The correlation of morphological and chemical characteristics. J Atheroscler Res. 1967 Mar-Apr;7(2):171–186. doi: 10.1016/s0368-1319(67)80079-6. [DOI] [PubMed] [Google Scholar]
- Svoboda D. J., Azarnoff D. L. Response of hepatic microbodies to a hypolipidemic agent, ethyl chlorophenoxyisobutyrate (CPIB). J Cell Biol. 1966 Aug;30(2):442–450. doi: 10.1083/jcb.30.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi S., Kamio A., Kubota I., Taura S. Ultrastructural aspects of the role of the media-smooth muscle cells in arteriosclerosis of man and animals. Acta Pathol Jpn. 1972 Nov;22(4):697–721. doi: 10.1111/j.1440-1827.1972.tb00756.x. [DOI] [PubMed] [Google Scholar]
- Wolinsky H., Goldfischer S., Schiller B., Kasak L. E. Lysosomes in aortic smooth muscle cells. Effects of hypertension. Am J Pathol. 1973 Dec;73(3):727–734. [PMC free article] [PubMed] [Google Scholar]