Abstract
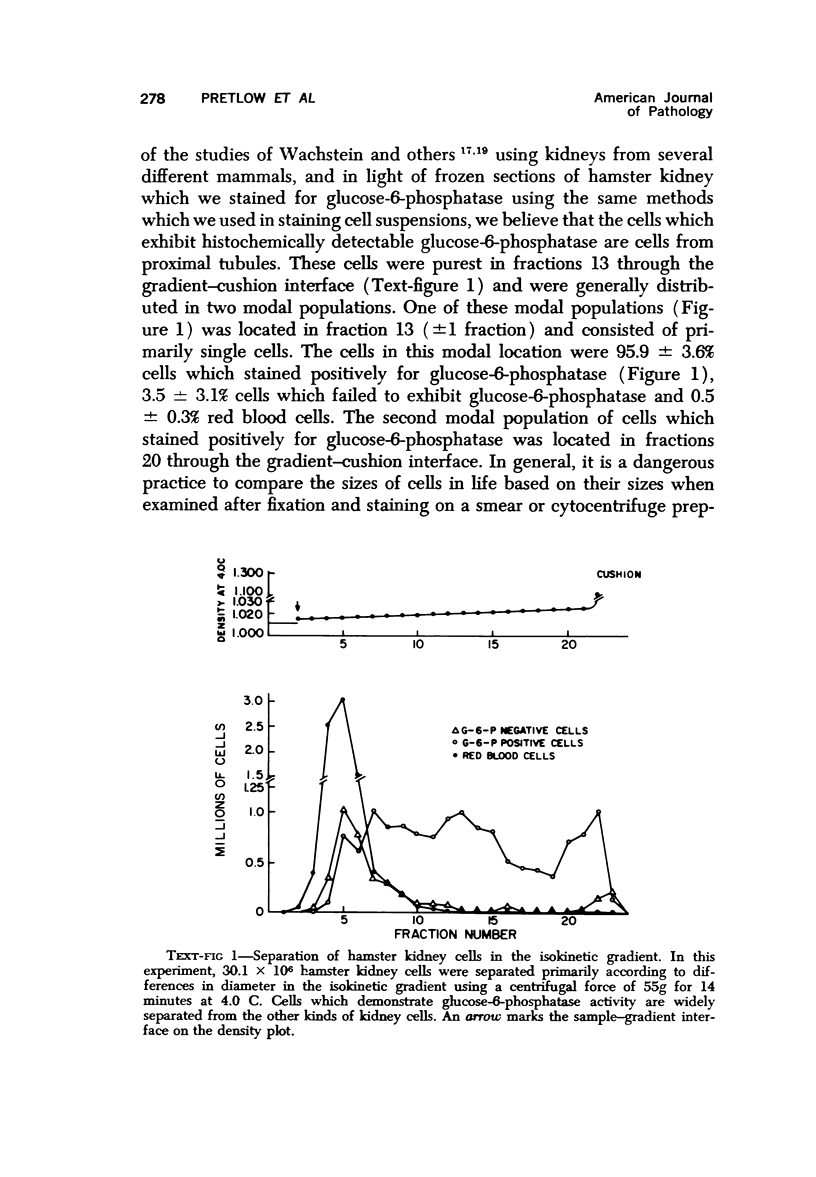
Suspensions of enzymatically disaggregated hamster kidney cells were separated primarily according to differences in diameter using velocity sedimentation in a previously described isokinetic density gradient and according to differences in density in an isopycnic density gradient. Cells which contained histochemically demonstrable glucose-6-phosphatase were thought to be cells from proximal tubules and constituted 46.5 ± 14.1% of the cells in the starting sample suspension of disaggregated kidney cells. The purest gradient fractions from experiments using velocity sedimentation contained 98.0 ± 0.6% cells which demonstrated glucose-6-phosphatase activity. More than 99.0% of these cells excluded trypan blue. Isopycnic sedimentation was not an effective means of purifying proximal tubule cells.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON N. G. Studies on isolated cell components. VIII. High resolution gradient differential centrifugation. Exp Cell Res. 1955 Dec;9(3):446–459. doi: 10.1016/0014-4827(55)90075-6. [DOI] [PubMed] [Google Scholar]
- BURG M. B., ORLOFF J. Oxygen consumption and active transport in separated renal tubules. Am J Physiol. 1962 Aug;203:327–330. doi: 10.1152/ajplegacy.1962.203.2.327. [DOI] [PubMed] [Google Scholar]
- Beard M. E., Novikoff A. B. Distribution of peroxisomes (microbodies) in the nephron of the rat: a cytochemical study. J Cell Biol. 1969 Aug;42(2):501–518. doi: 10.1083/jcb.42.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. J., Sacktor B. Isolation and biochemical characterization of brush borders from rabbit kidney. J Cell Biol. 1970 Dec;47(3):637–645. doi: 10.1083/jcb.47.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmon J., Pitts A., Pretlow T. G., 2nd Separation of lipase-positive cells from suspensions of pancreas cells in an isokinetic gradient of Ficoll in tissue culture medium. Am J Pathol. 1973 Sep;72(3):417–426. [PMC free article] [PubMed] [Google Scholar]
- Bondi E. E., Devlin T. M., Ch'ih J. J. Distribution of two mitochondrial populations in rabbit kidney cortex and medulla. Biochem Biophys Res Commun. 1972 May 12;47(3):574–580. doi: 10.1016/0006-291x(72)90917-5. [DOI] [PubMed] [Google Scholar]
- Burlington H., Cronkite E. P., Reincke U., Zanjani E. D. Erythropoietin production in cultures of goat renal glomeruli. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3547–3550. doi: 10.1073/pnas.69.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade-Treyer D. Isolation of pure fractions of viable calf kidney tubules and glomeruli. "In vitro" culture, immunochemical and esterase zymogram analysis. Ann Inst Pasteur (Paris) 1972 Feb;122(2):263–281. [PubMed] [Google Scholar]
- Chesney R. W., Sacktor B., Rowen R. The binding of D-glucose to the isolated luminal membrane of the renal proximal tubule. J Biol Chem. 1973 Mar 25;248(6):2182–2191. [PubMed] [Google Scholar]
- Choie D. D., Richter G. W. Cell proliferation in rat kidney induced by lead acetate and effects of uninephrectomy on the proliferation. Am J Pathol. 1972 Feb;66(2):265–275. [PMC free article] [PubMed] [Google Scholar]
- Dawson A. G. Preparation and some properties of a suspension of fragmented tubules from rat kidney. Biochem J. 1972 Nov;130(2):525–532. doi: 10.1042/bj1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsas L. J., Hayslett J. P., Spargo B. H., Durant J. L., Rosenberg L. E. Wilson's disease with reversible renal tubular dysfunction. Correlation with proximal tubular ultrastructure. Ann Intern Med. 1971 Sep;75(3):427–433. doi: 10.7326/0003-4819-75-3-427. [DOI] [PubMed] [Google Scholar]
- GREENE H. S. The heterologous transplantation of human testicular tumors. Cancer Res. 1954 Aug;14(7):516–518. [PubMed] [Google Scholar]
- GREENSPON S. A., KRAKOWER C. A. Direct evidence for the antigenicity of the glomeruli in the production of nephrotoxic serums. AMA Arch Pathol. 1950 Mar;49(3):291–297. [PubMed] [Google Scholar]
- Heidrich H. G., Kinne R., Kinne-Saffran E., Hannig K. The polarity of the proximal tubule cell in rat kidney. Different surface charges for the brush-border microvilli and plasma membranes from the basal infoldings. J Cell Biol. 1972 Aug;54(2):232–245. doi: 10.1083/jcb.54.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON L. O., GOLDWASSER E., FRIED W., PLZAK L. Role of the kidney in erythropoiesis. Nature. 1957 Mar 23;179(4560):633–634. doi: 10.1038/179633a0. [DOI] [PubMed] [Google Scholar]
- Jacobson N. O., Jorgensen F., Thomsen A. C. On the localization of some phosphatases in three different segments of the proximal tubules in the rat kidney. J Histochem Cytochem. 1967 Aug;15(8):456–469. doi: 10.1177/15.8.456. [DOI] [PubMed] [Google Scholar]
- Kaplan B. S., Drummond K. N. Glucose metabolism by isolated rat glomeruli in hyperproteinemic proteinuria. Lab Invest. 1972 Dec;27(6):590–593. [PubMed] [Google Scholar]
- LONGLEY J. B., FISHER E. R. Alkaline phosphatase and the periodic acid Schiff reaction in the proximal tubule of the vertebrate kidney; a study in segmental differentiation. Anat Rec. 1954 Sep;120(1):1–21. doi: 10.1002/ar.1091200102. [DOI] [PubMed] [Google Scholar]
- Mattenheimer H., Pollak V. E., Muehrcke R. C. Quantitative enzyme patterns in the nephron of the healthy human kidney. Nephron. 1970;7(2):144–154. doi: 10.1159/000179816. [DOI] [PubMed] [Google Scholar]
- Misra R. P. Isolation of glomeruli from mammalian kidneys by graded sieving. Am J Clin Pathol. 1972 Aug;58(2):135–139. doi: 10.1093/ajcp/58.2.135. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W. Separation of mammalian cells using programmed gradient sedimentation. Exp Mol Pathol. 1969 Oct;11(2):139–152. doi: 10.1016/0014-4800(69)90003-3. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Glick M. R., Reddy W. J. Separation of beating cardiac myocytes from suspensions of heart cells. Am J Pathol. 1972 May;67(2):215–226. [PMC free article] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Williams E. E. Separation of hepatocytes from suspensions of mouse liver cells using programmed gradient sedimentation in gradients of ficoll in tissue sulture medium. Anal Biochem. 1973 Sep;55(1):114–122. doi: 10.1016/0003-2697(73)90296-0. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G. Estimation of experimental conditions that permit cell separations by velocity sedimentation on isokinetic gradients of Ficoll in tissue culture medium. Anal Biochem. 1971 May;41(1):248–255. doi: 10.1016/0003-2697(71)90207-7. [DOI] [PubMed] [Google Scholar]
- Quadracci L., Striker G. E. Growth and maintenance of glomerular cells in vitro. Proc Soc Exp Biol Med. 1970 Dec;135(3):947–950. doi: 10.3181/00379727-135-35177. [DOI] [PubMed] [Google Scholar]
- STERNBERG W. H., FARBER E., DUNLAP C. E. Histochemical localization of specific oxidative enzymes. II. Localization of diphosphopyridine nucleotide and triphosphopyridine nucleotide diaphorases and the succindehydrogenase system in the kidney. J Histochem Cytochem. 1956 May;4(3):266–283. doi: 10.1177/4.3.266. [DOI] [PubMed] [Google Scholar]
- Shimamura T., Morrison A. B. Vascular permeability of the renal medullary vessels in the mouse and rat. Am J Pathol. 1973 May;71(2):155–163. [PMC free article] [PubMed] [Google Scholar]
- Trump B. F., Valigorsky J. M., Dees J. H., Mergner W. J., Kim K. M., Jones R. T., Pendergrass R. E., Garbus J., Cowley R. A. Cellular change in human disease. A new method of pathological analysis. Hum Pathol. 1973 Mar;4(1):89–109. doi: 10.1016/s0046-8177(73)80050-4. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., BRADSHAW M. HISTOCHEMICAL LOCALIZATION OF ENZYME ACTIVITY IN THE KIDNEYS OF THREE MAMMALIAN SPECIES DURING THEIR POSTNATAL DEVELOPMENT. J Histochem Cytochem. 1965 Jan;13:44–56. doi: 10.1177/13.1.44. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M. Histochemical staining reactions of the normally functioning and abnormal kidney. J Histochem Cytochem. 1955 Jul;3(4):246–270. doi: 10.1177/3.4.246. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Wilson H., Spargo B., Getz G. S. Changes in kidney medullary phospholipid metabolism in the potassium-deficient rat. I. Am J Pathol. 1973 May;71(2):295–314. [PMC free article] [PubMed] [Google Scholar]
- Wilson H., Spargo B., Getz G. S. Changes in kidney medullary phospholipid metabolism in the potassium-depleted rat. II. Am J Pathol. 1973 May;71(2):315–330. [PMC free article] [PubMed] [Google Scholar]