Abstract
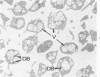
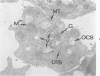
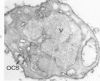
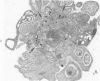
Phorbol myristate acetate (PMA), the active principle of croton oil, is a potent platelet aggregating agent. A previous electron microscopic study indicated that PMA caused selective labilization of platelet granules. The present investigation has employed cytochemical procedures to clarify problems concerning the action of PMA on platelets. Results of this study confirm the suggestion that vacuoles in PMA-treated platelets derive primarily from granules, and demonstrate that the swollen vacuoles are continuous with channels of the open canalicular system (OCS) and surrounding plasma. Despite loss of the barrier separating vacuolated granules from the OCS, the contents of the organelles were not extruded from the PMA aggregated platelets. Stabilization of platelet surface membranes did not inhibit the action of PMA on platelet granules, and examination of replicas of freeze-fractured PMA platelets failed to reveal any specific injury produced by the agent. The findings have elucidated some of the effects of PMA on platelet structure, but have not solved the basic mechanism of drug action.
Full text
PDF










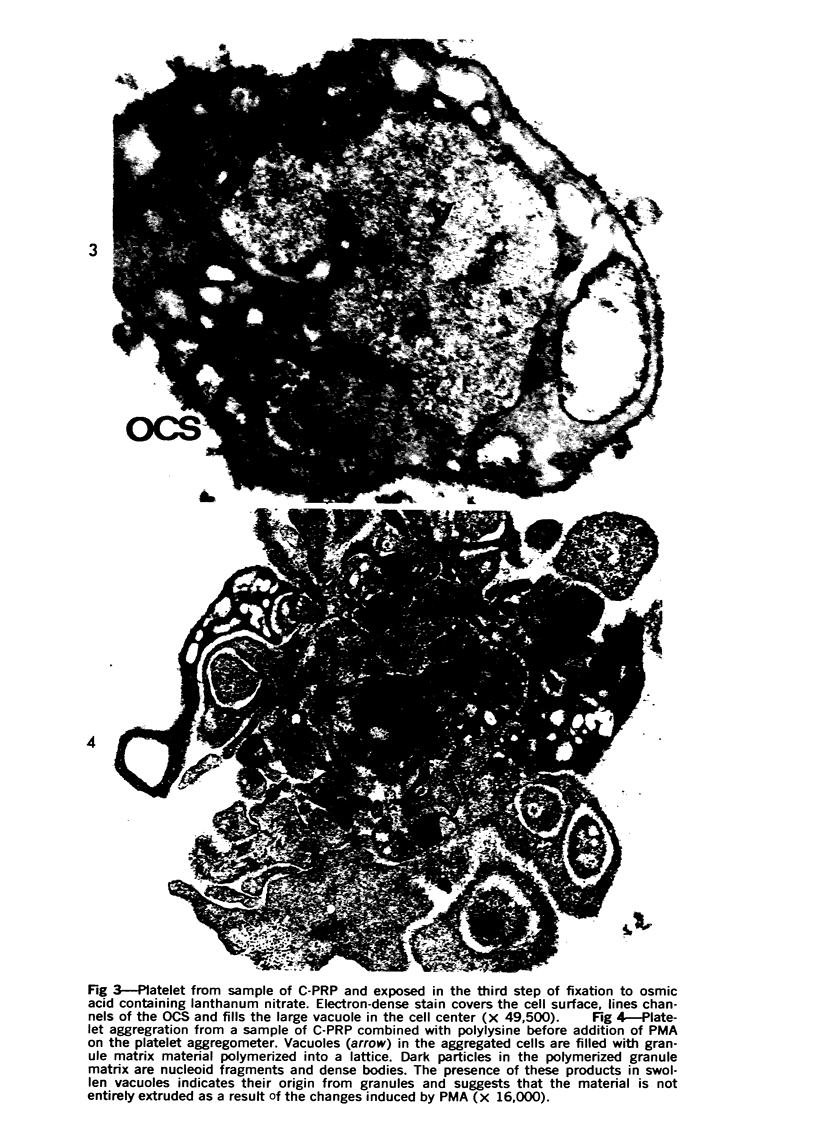
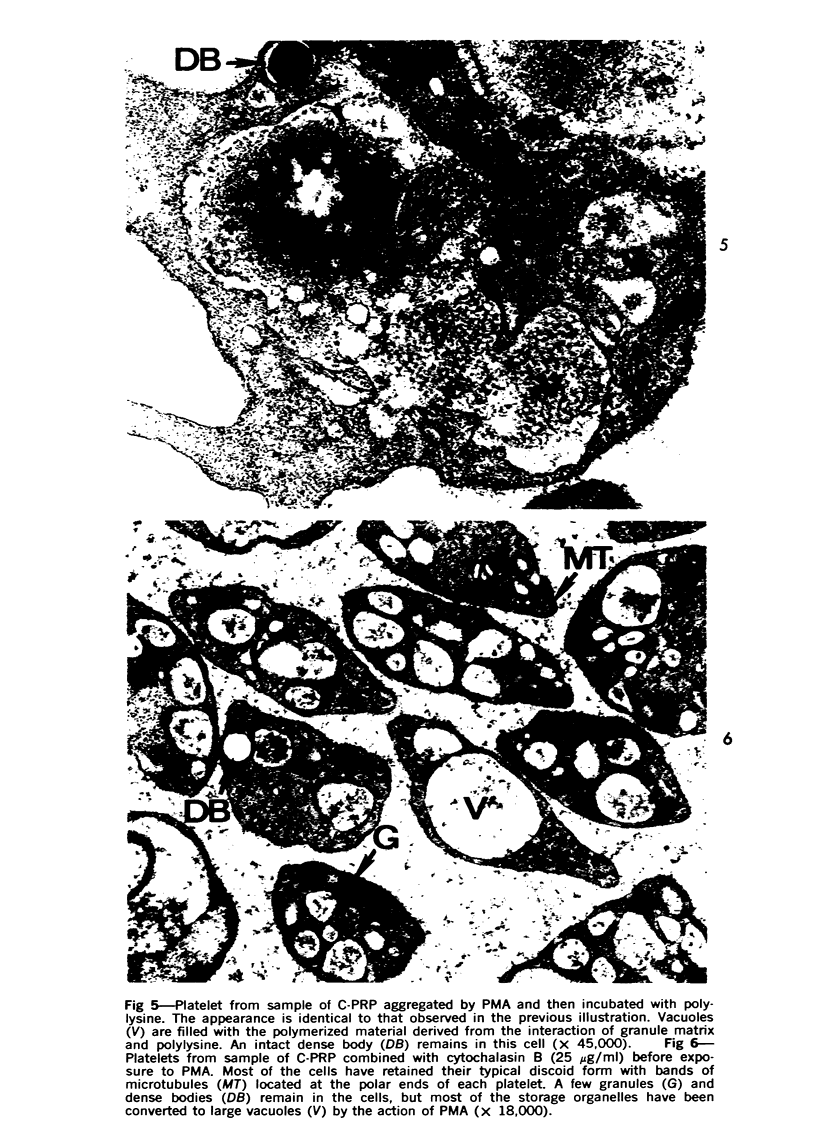

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Estensen R. D., White J. G. Ultrastructural features on the platelet response to phorbol myristate acetate. Am J Pathol. 1974 Mar;74(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Stormorken H. The blood platelet release reaction. Scand J Haematol Suppl. 1969;8:3–26. [PubMed] [Google Scholar]
- Weissmann G., Troll W., Van Duuren B. L., Sessa G. Studies on lysosomes. X. Effects of tumor-promoting agents upon biological and artificial membrane systems. Biochem Pharmacol. 1968 Dec;17(12):2421–2434. doi: 10.1016/0006-2952(68)90132-9. [DOI] [PubMed] [Google Scholar]
- White J. G. A search for the platelet secretory pathway using electron dense tracers. Am J Pathol. 1970 Jan;58(1):31–49. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Conard W. J. The fine structure of freeze-fractured blood platelets. Am J Pathol. 1973 Jan;70(1):45–56. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Exocytosis of secretory organelles from blood platelets incubated with cationic polypeptides. Am J Pathol. 1972 Oct;69(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- White J. G. Interaction of membrane systems in blood platelets. Am J Pathol. 1972 Feb;66(2):295–312. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Krumwiede M. Influence of cytochalasin B on the shape change induced in platelets by cold. Blood. 1973 Jun;41(6):823–832. [PubMed] [Google Scholar]
- White J. G. The transfer of thorium particles from plasma to platelets and platelet granules. Am J Pathol. 1968 Oct;53(4):567–575. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Uptake of latex particles by blood platelets: phagocytosis or sequestration? Am J Pathol. 1972 Dec;69(3):439–458. [PMC free article] [PubMed] [Google Scholar]