Abstract
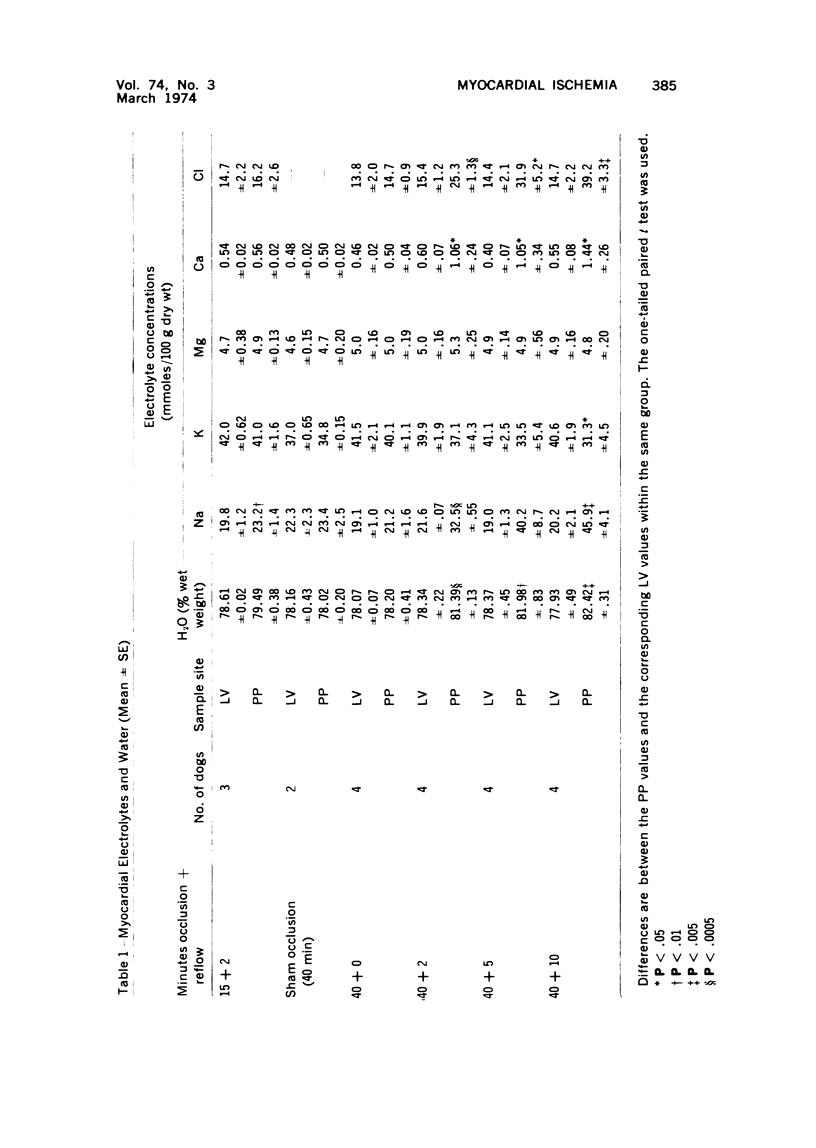
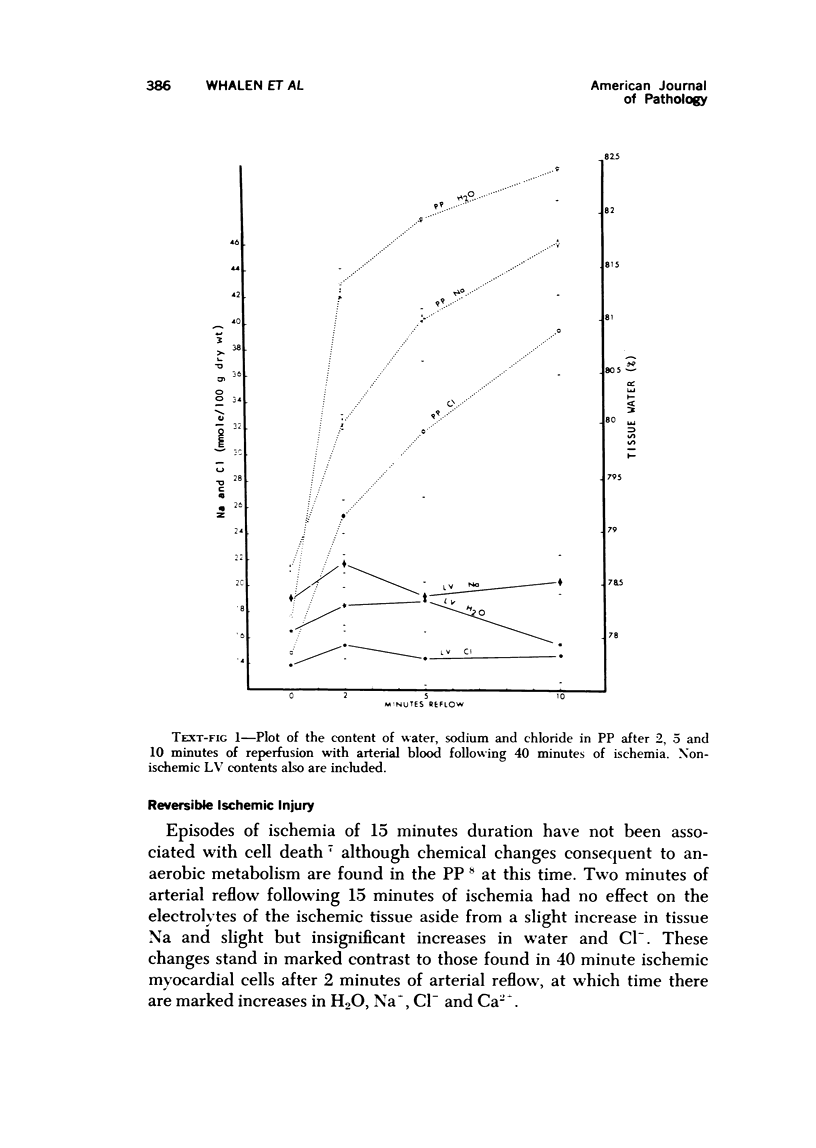
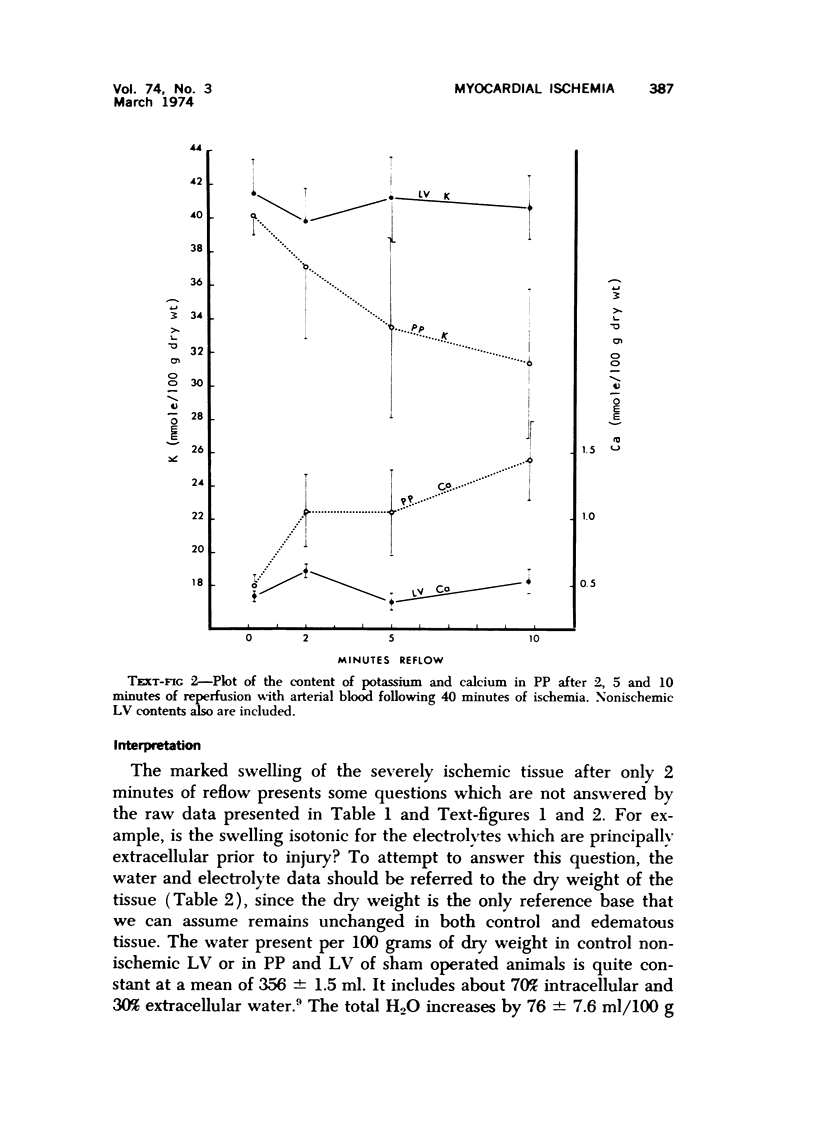
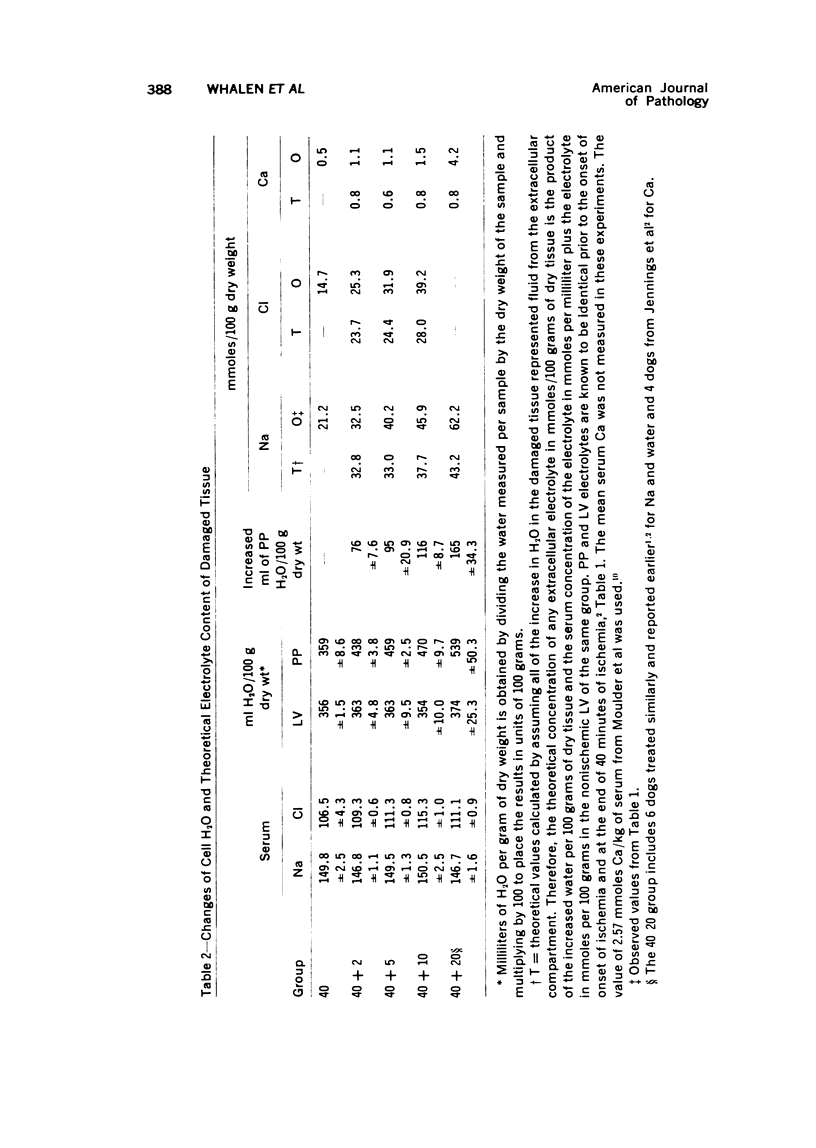
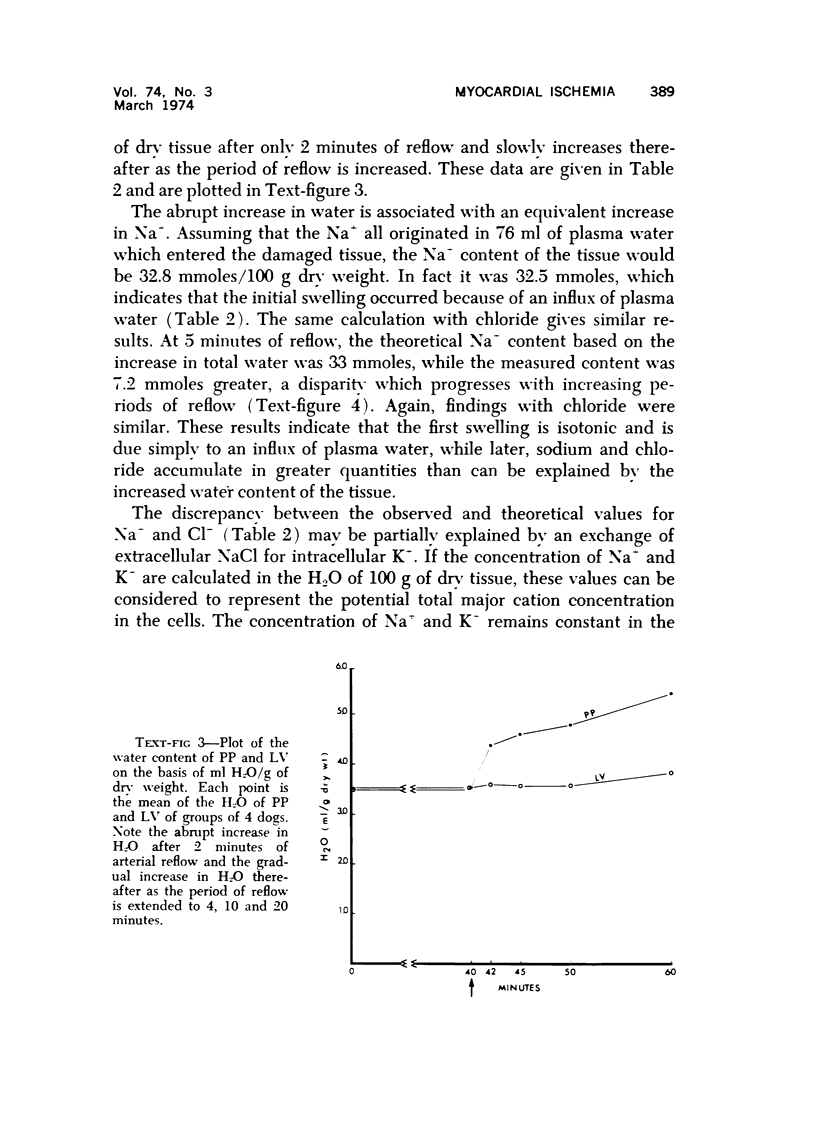
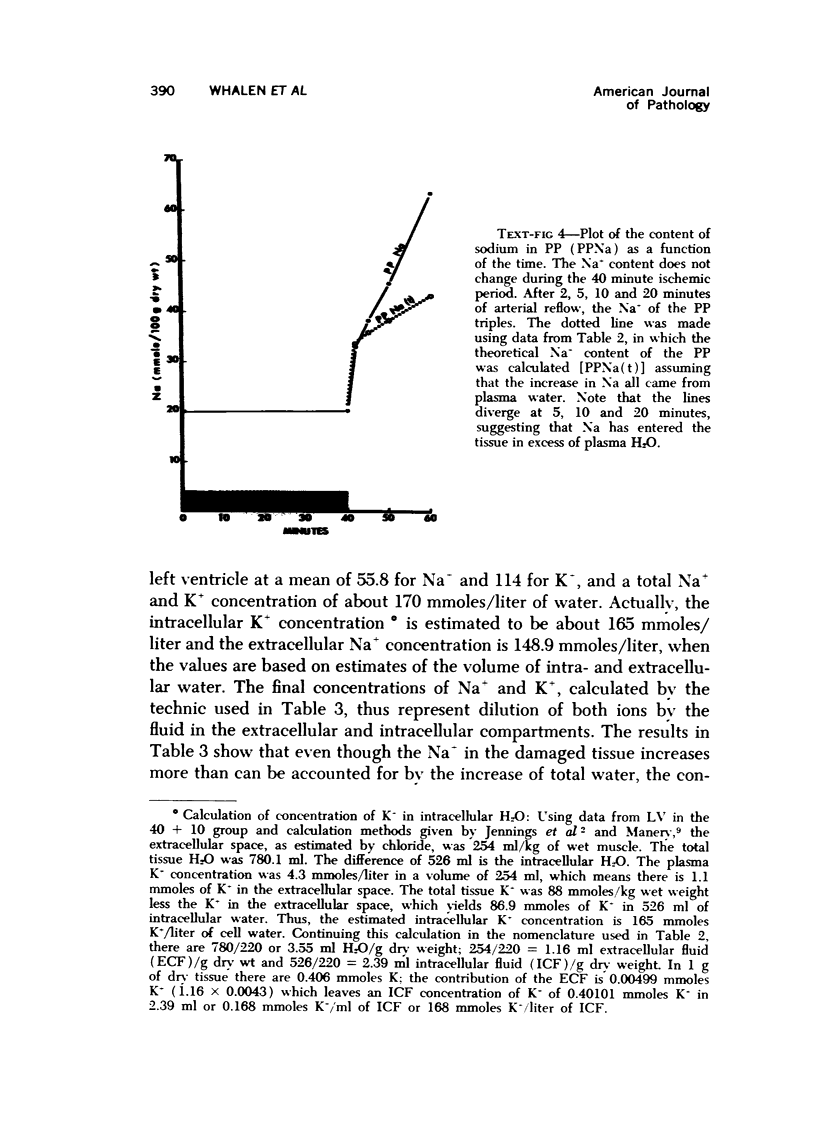
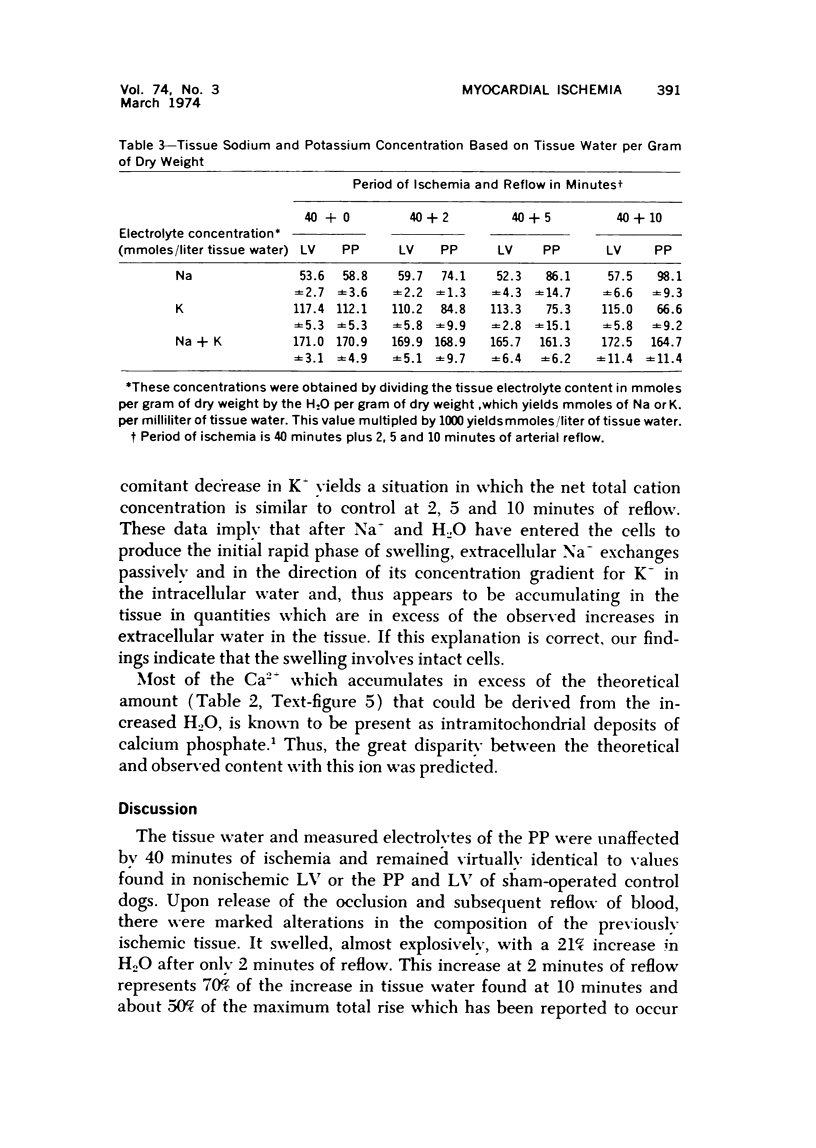
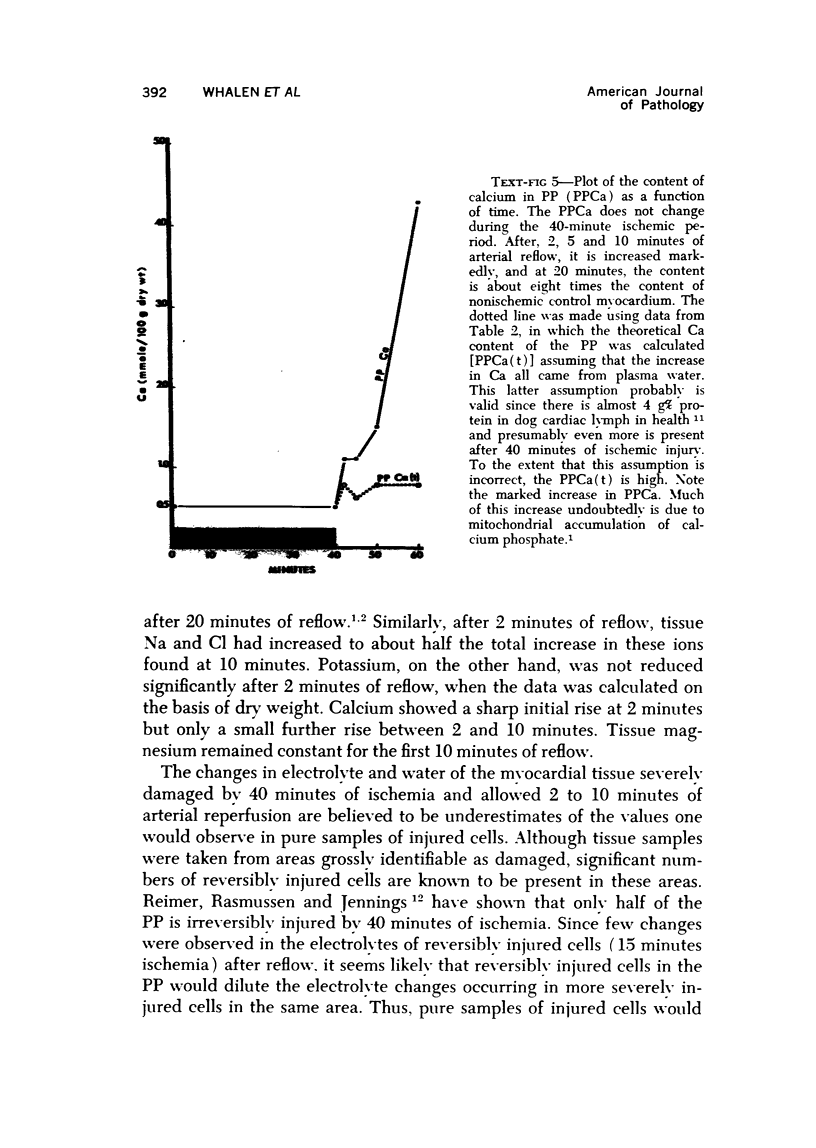
The effect of temporary periods of ischemia on the electrolytes and water of myocardial cells were studied in groups of mongrel dogs. Myocardial tissue exposed to 40 minutes of ischemia induced by occlusion of the circumflex branch of the left coronary artery developed no changes in water or electrolytes when compared to nonischemic left ventricle of the same or sham-operated animals, even though this period of ischemia is known to produce irreversible injury to many of the damaged cells. However, reperfusion of the affected myocardium with arterial blood for only 2 minutes resulted in striking increases in tissue H2O, Na-, Cl- and Ca2-. These changes in electrolytes increased in severity with longer periods of reflow, and tissue K+ was decreased significantly after 10 minutes of reflow had passed. Analysis of the results suggested that the tissue edema was primarily the result of cellular swelling. Myocardium exposed to 15 minutes of ischemia followed by 2 minutes of reflow showed no significant changes aside from a slight increase in Na+. These studies demonstrate that defects in cell volume regulation occur early in severe ischemic injury.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braasch W., Gudbjarnason S., Puri P. S., Ravens K. G., Bing R. J. Early changes in energy metabolism in the myocardium following acute coronary artery occlusion in anesthetized dogs. Circ Res. 1968 Sep;23(3):429–438. doi: 10.1161/01.res.23.3.429. [DOI] [PubMed] [Google Scholar]
- CONN H. L., Jr, WOOD J. C., MORALES G. S. Rate of change in myocardial glycogen and lactic acid following arrest of coronary circulation. Circ Res. 1959 Sep;7:721–727. doi: 10.1161/01.res.7.5.721. [DOI] [PubMed] [Google Scholar]
- Herdson P. B., Kaltenbach J. P., Jennings R. B. Fine structural and biochemical changes in dog myocardium during autolysis. Am J Pathol. 1969 Dec;57(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- JENNINGS R. B., SOMMERS H. M., KALTENBACH J. P., WEST J. J. ELECTROLYTE ALTERATIONS IN ACUTE MYOCARDIAL ISCHEMIC INJURY. Circ Res. 1964 Mar;14:260–269. doi: 10.1161/01.res.14.3.260. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Herdson P. B., Sommers H. M. Structural and functional abnormalities in mitochondria isolated from ischemic dog myocardium. Lab Invest. 1969 Jun;20(6):548–557. [PubMed] [Google Scholar]
- Jennings R. B., Moore C. B., Shen A. C., Herdson P. B. Electrolytes of damaged myocardial mitochondria. Proc Soc Exp Biol Med. 1970 Nov;135(2):515–522. doi: 10.3181/00379727-135-35086. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Kübler W., Spieckermann P. G. Regulation of glycolysis in the ischemic and the anoxic myocardium. J Mol Cell Cardiol. 1970 Dec;1(4):351–377. doi: 10.1016/0022-2828(70)90034-9. [DOI] [PubMed] [Google Scholar]
- Leaf A. Regulation of intracellular fluid volume and disease. Am J Med. 1970 Sep;49(3):291–295. doi: 10.1016/s0002-9343(70)80019-5. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANERY J. F. Water and electrolyte metabolism. Physiol Rev. 1954 Apr;34(2):334–417. doi: 10.1152/physrev.1954.34.2.334. [DOI] [PubMed] [Google Scholar]
- Moulder P. V., Eichelberger L., Rams J. J., Greenburg A. G. Water, nitrogen, and electrolyte content of right and left ventricular walls and interventricular septum of normal canine hearts. Circ Res. 1966 Sep;19(3):662–667. doi: 10.1161/01.res.19.3.662. [DOI] [PubMed] [Google Scholar]
- Page E., Polimeni P. I. Magnesium exchange in rat ventricle. J Physiol. 1972 Jul;224(1):121–139. doi: 10.1113/jphysiol.1972.sp009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer K. A., Rasmussen M. M., Jennings R. B. Reduction by propranolol of myocardial necrosis following temporary coronary artery occlusion in dogs. Circ Res. 1973 Sep;33(3):353–363. doi: 10.1161/01.res.33.3.353. [DOI] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Kinetics of calcium accumulation in acute myocardial ischemic injury. Am J Pathol. 1972 Jun;67(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]


