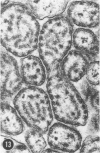
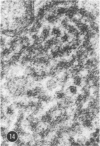
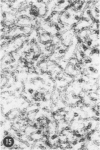
Abstract
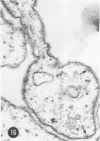
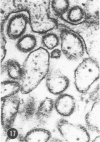
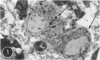
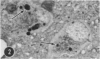
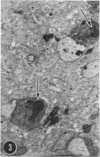
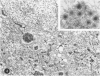
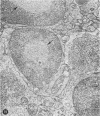
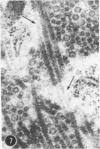
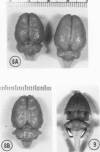
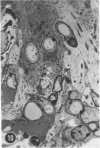
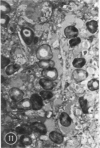
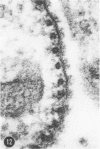
A series of experiments has been described in which litters of suckling rats were inoculated either with wild-type reovirus type III or one of two of its temperature-sensitive (ts) mutants. While the wild-type virus produced an acute, fatal syndrome, the ts mutants were substantially less neurovirulent. Of the ts mutant-inoculated animals, a large percentage of the surviving (chronic) animals given ts mutant B showed an unobstructive hydrocephalus ex vacuo whereas chronic ts mutant C animals showed no visible nervous system disease. The ts mutants persisted within the central nervous system (CNS) for 6 to 8 weeks, after which they could not be detected either virologically, immunologically or morphologically. In another set of experiments, organized CNS explants were studied following infection with either measles virus or the neuroadapted Mantooth strain of SSPE virus, a variant of measles. Wild measles (Edmonston strain) exerted an acute destructive effect, but SSPE virus had a tendency to enter into coexistence with the tissue without destroying its organotypic nature. These relationships are somewhat reminiscent of the neuropathologic conditions caused by these two viruses in man. Since the reovirus type III ts mutants possess both genetic and morphologic defects and in many instances cause CNS conditions different from that induced by the wild-type virus, it has been proposed that a comparable situation may exist after measles and SSPE virus infection. SSPE virions of the strain studied were found to be defective in certain viral components which may have contributed to the lower neurovirulence and its entering into a chronic relationship with the CNS, in contrast to the acute destructive nature of measles infection. The findings are discussed in terms of relevance to other chronic CNS diseases, particularly multiple sclerosis, in which the possiblity exists that a mutant virus is operative.
Full text
PDF













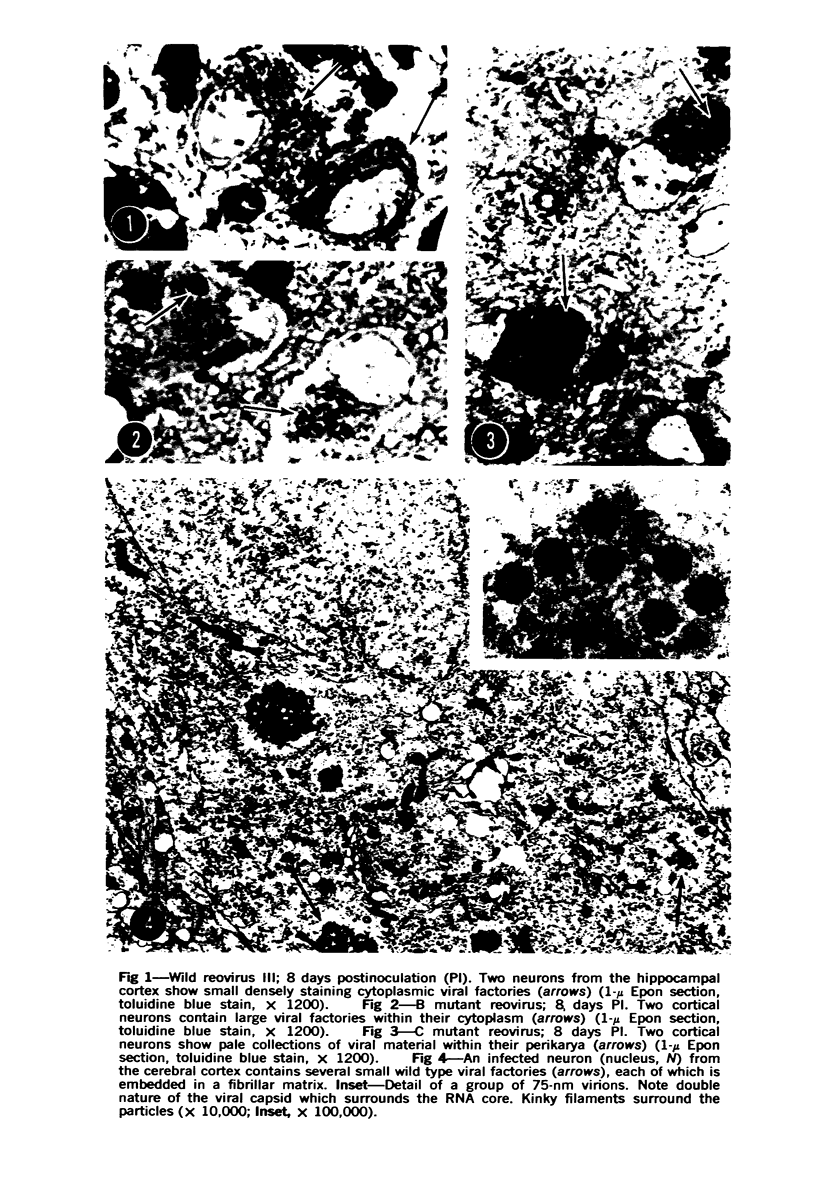
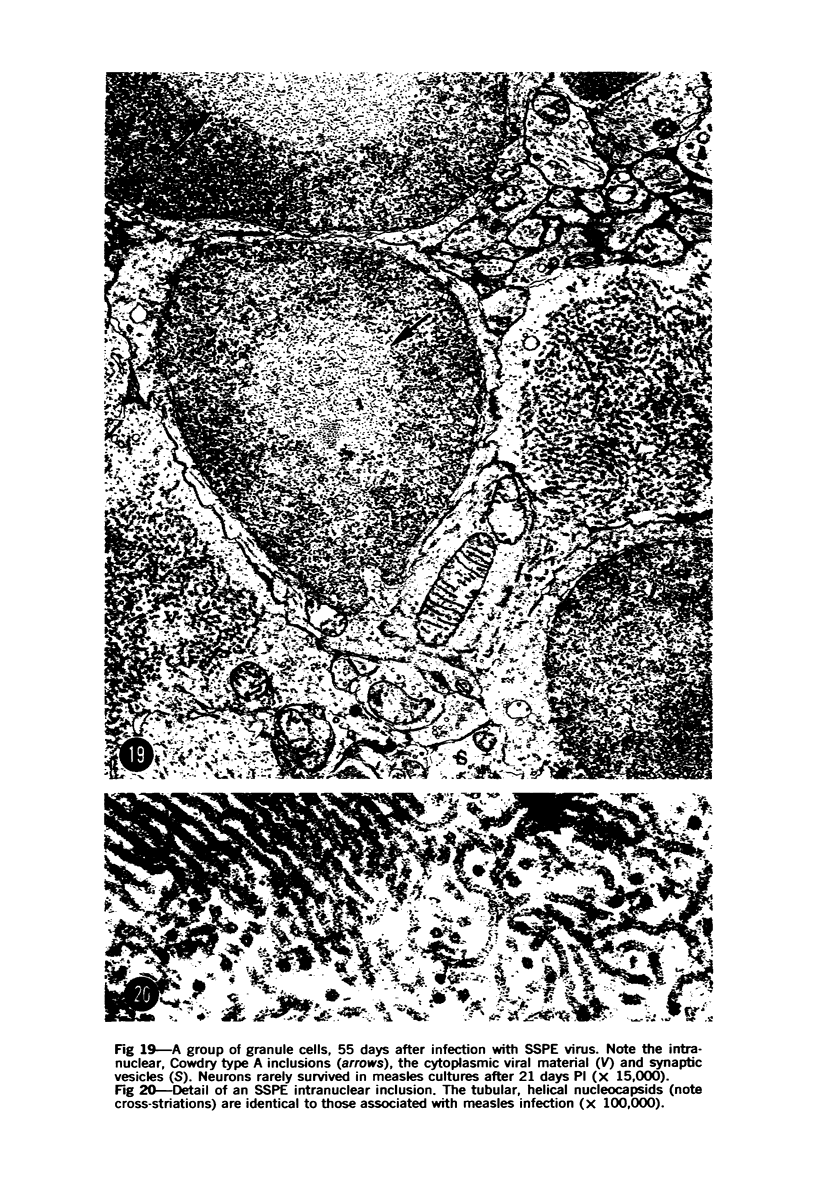
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS J. M., IMAGAWA D. T. Measles antibodies in multiple sclerosis. Proc Soc Exp Biol Med. 1962 Dec;111:562–566. doi: 10.3181/00379727-111-27855. [DOI] [PubMed] [Google Scholar]
- Albrecht P., Schumacher H. P. Neurotropic properties of measles virus in hamsters and mice. J Infect Dis. 1971 Jul;124(1):86–93. doi: 10.1093/infdis/124.1.86. [DOI] [PubMed] [Google Scholar]
- Albrecht P., Shabo A. L., Burns G. R., Tauraso N. M. Experimental measles encephalitis in normal and cyclophosphamide-treated rhesus monkeys. J Infect Dis. 1972 Aug;126(2):154–161. doi: 10.1093/infdis/126.2.154. [DOI] [PubMed] [Google Scholar]
- BORNSTEIN M. B., MURRAY M. R. Serial observations on patterns of growth, myelin formation, maintenance and degeneration in cultures of new-born rat and kitten cerebellum. J Biophys Biochem Cytol. 1958 Sep 25;4(5):499–504. doi: 10.1083/jcb.4.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Byington D. P., Castro A. E., Burnstein T. Adaptation to hamsters of neurotropic measles virus from subacute sclerosing panencephalitis. Nature. 1970 Feb 7;225(5232):554–555. doi: 10.1038/225554b0. [DOI] [PubMed] [Google Scholar]
- Byington D. P., Johnson K. P. Experimental subacute sclerosing panencephalitis in the hamster: correlation of age with chronic inclusion-cell encephalitis. J Infect Dis. 1972 Jul;126(1):18–26. doi: 10.1093/infdis/126.1.18. [DOI] [PubMed] [Google Scholar]
- Chen T. T., Watanabe I., Zeman W., Mealey J., Jr Subacute sclerosing panencephalitis: propagation of measles virus from brain biopsy in tissue culture. Science. 1969 Mar 14;163(3872):1193–1194. doi: 10.1126/science.163.3872.1193. [DOI] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral RNA. Virology. 1972 Dec;50(3):799–809. doi: 10.1016/0042-6822(72)90434-5. [DOI] [PubMed] [Google Scholar]
- DALES S. ASSOCIATION BETWEEN THE SPINDLE APPARATUS AND REOVIRUS. Proc Natl Acad Sci U S A. 1963 Aug;50:268–275. doi: 10.1073/pnas.50.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., GOMATOS P. J., HSU K. C. THE UPTAKE AND DEVELOPMENT OF REOVIRUS IN STRAIN L CELLS FOLLOWED WITH LABELED VIRAL RIBONUCLEIC ACID AND FERRITIN-ANTIBODY CONJUGATES. Virology. 1965 Feb;25:193–211. doi: 10.1016/0042-6822(65)90199-6. [DOI] [PubMed] [Google Scholar]
- Feldman L. A., Raine C. S., Sheppard R. D., Bornstein M. B. Virus-host cell relationships in measles-infected cultures of central nervous tissue. J Neuropathol Exp Neurol. 1972 Oct;31(4):624–638. doi: 10.1097/00005072-197210000-00006. [DOI] [PubMed] [Google Scholar]
- Fields B. N. Genetic manipulation of reovirus--a model for modification of disease. N Engl J Med. 1972 Nov 16;287(20):1026–1033. doi: 10.1056/NEJM197211162872007. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Laskov R., Scharff M. D. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral peptides. Virology. 1972 Oct;50(1):209–215. doi: 10.1016/0042-6822(72)90361-3. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Raine C. S., Baum S. G. Temperature-sensitive mutants of reovirus type 3: defects in viral maturation as studied by immunofluorescence and electron microscopy. Virology. 1971 Mar;43(3):569–578. doi: 10.1016/0042-6822(71)90282-0. [DOI] [PubMed] [Google Scholar]
- Fields B. N. Temperature-sensitive mutants of reovirus type 3 features of genetic recombination. Virology. 1971 Oct;46(1):142–148. doi: 10.1016/0042-6822(71)90013-4. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Margolis G., Kilham L. Reovirus type 3 encephalitis: observations of virus-cell interactions in neural tissues. II. Electron microscopic studies. Lab Invest. 1971 Feb;24(2):101–109. [PubMed] [Google Scholar]
- Grose C., Feorino P. M. Epstein-Barr virus and Guillain-Barré syndrome. Lancet. 1972 Dec 16;2(7790):1285–1287. doi: 10.1016/s0140-6736(72)92654-2. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Byington D. P. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. I. Acute giant cell encephalitis in newborn animals. Exp Mol Pathol. 1971 Dec;15(3):373–379. doi: 10.1016/0014-4800(71)90044-x. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Klasnja R., Johnson R. T. Neural tube defects of chick embryos: an indirect result of influenza A virus infection. J Neuropathol Exp Neurol. 1971 Jan;30(1):68–74. doi: 10.1097/00005072-197101000-00007. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus as a sequela of experimental myxovirus infections. Exp Mol Pathol. 1969 Feb;10(1):68–80. doi: 10.1016/0014-4800(69)90049-5. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus following viral infection: the pathology of aqueductal stenosis developing after experimental mumps virus infection. J Neuropathol Exp Neurol. 1968 Oct;27(4):591–606. [PubMed] [Google Scholar]
- Johnson R. T. Mumps virus encephalitis in the hamster. Studies of the inflammatory response and noncytopathic infection of neurons. J Neuropathol Exp Neurol. 1968 Jan;27(1):80–95. doi: 10.1097/00005072-196801000-00005. [DOI] [PubMed] [Google Scholar]
- Katz M., Oyanagi S., Koprowski H. Subacute sclerosing panencephalitis: structures resembling myxovirus nucleocapsids in cells cultured from brain. Nature. 1969 May 31;222(5196):888–890. doi: 10.1038/222888a0. [DOI] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. I. Virologic studies. Lab Invest. 1969 Sep;21(3):183–188. [PubMed] [Google Scholar]
- Margolis G., Kilham L., Gonatas N. K. Reovirus type 3 encephalitis: observations of virus-cell interactions in neural tissues. I. Light microscopy studies. Lab Invest. 1971 Feb;24(2):91–100. [PubMed] [Google Scholar]
- Margolis G., Kilham L. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. II. Pathologic studies. Lab Invest. 1969 Sep;21(3):189–198. [PubMed] [Google Scholar]
- Nakai M., Imagawa D. T. Electron microscopy of measles virus replication. J Virol. 1969 Feb;3(2):187–197. doi: 10.1128/jvi.3.2.187-197.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. L., Baringer J. R. Reovirus-induced aqueductal stenosis in hamsters. Phase contrast and electron microscopic studies. Lab Invest. 1972 Dec;27(6):531–537. [PubMed] [Google Scholar]
- Oyanagi S., ter Meulen V., Katz M., Koprowski H. Comparison of subacute sclerosing panencephalitis and measles viruses: an electron microscope study. J Virol. 1971 Jan;7(1):176–187. doi: 10.1128/jvi.7.1.176-187.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas J. Paramyxovirus-like particles associated with acute demyelination in chronic relapsing multiple sclerosis. Science. 1972 Nov 17;178(4062):760–763. doi: 10.1126/science.178.4062.760. [DOI] [PubMed] [Google Scholar]
- RAPP F. PLAQUE DIFFERENTIATION AND REPLICATION OF VIRULENT AND ATTENUATED STRAINS OF MEASLES VIRUS. J Bacteriol. 1964 Nov;88:1448–1458. doi: 10.1128/jb.88.5.1448-1458.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Subacute sclerosing panencephalitis virus in cultures of organized central nervous tissue. Lab Invest. 1973 May;28(5):627–640. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructure of measles virus in cultures of hamster cerebellum. J Virol. 1969 Aug;4(2):169–181. doi: 10.1128/jvi.4.2.169-181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Neurotropic viruses and the developing brain. N Y State J Med. 1973 May 15;73(10):1169–1179. [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Reovirus type 3 encephalitis--a virologic and ultrastructural study. J Neuropathol Exp Neurol. 1973 Jan;32(1):19–33. doi: 10.1097/00005072-197301000-00002. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Powers J. M., Suzuki K. Acute multiple sclerosis. Confirmation of "paramyxovirus-like" intranuclear inclusions. Arch Neurol. 1974 Jan;30(1):39–46. doi: 10.1001/archneur.1974.00490310041007. [DOI] [PubMed] [Google Scholar]
- Rawls W. E. Congenital rubella: the significance of virus persistence. Prog Med Virol. 1968;10:238–285. [PubMed] [Google Scholar]
- WALTERS M. N., JOSKE R. A., LEAK P. J., STANLEY N. F. MURINE INFECTION WITH REOVIRUS: I. PATHOLOGY OF THE ACUTE PHASE. Br J Exp Pathol. 1963 Aug;44:427–436. [PMC free article] [PubMed] [Google Scholar]
- WATERSON A. P. MEASLES VIRUS. Arch Gesamte Virusforsch. 1965;16:57–80. doi: 10.1007/BF01253793. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Okazaki H. Virus-like structure in multiple sclerosis. Lancet. 1973 Sep 8;2(7828):569–570. doi: 10.1016/s0140-6736(73)92394-5. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Herndon R. M., Narayan O., Johnson R. T., Shah K., Rubinstein L. J., Preziosi T. J., Conley F. K. Isolation of virus related to SV40 from patients with progressive multifocal leukoencephalopathy. N Engl J Med. 1972 Feb 24;286(8):385–390. doi: 10.1056/NEJM197202242860801. [DOI] [PubMed] [Google Scholar]