Abstract
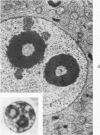
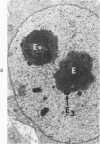
A strain of mouse adenovirus, found to have a striking tropism for the weanling mouse adrenal gland, enabled electron microscopic examination of adrenals in various stages of infection. Nucleolar hypertrophy and the successive formation of three types of inclusion bodies in association with nucleoli preceded virion production. Angular crystals of virions formed in the affected nuclei. Virus was released by lysis of nuclear membranes; rapid degeneration of cytoplasmic organelles followed. Rupture of external cell membranes released virus into the extracellular spaces where virions crossed vascular basement membranes to enter endothelial cells. Virions were also phagocytized by inflammatory cells which reentered vascular sinusoids, and by adrenal parenchymal cells. Disruption of virus-laden phagocytic vacuoles in parenchymal cells released virions into the cytoplasm. Typical viral inclusion bodies also formed in vascular endothelial cells and in inflammatory cells, but virion replication was not detected. The possibility that virus directly entered parenchymal cells through the external cell membrane without phagocytosis is discussed.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aterman K., Embil J., Easterbrook K. B., Haldane E. V., Crosby J. Liver necrosis, adenovirus type 2 and thymic dysplasia. Virchows Arch A Pathol Pathol Anat. 1973 Aug 9;360(2):155–171. doi: 10.1007/BF00543226. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Breynaert M. D., Biserte G. Lysosomes and the problem of adenovirus uncoating. Exp Mol Pathol. 1970 Apr;12(2):235–242. doi: 10.1016/0014-4800(70)90053-5. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Burlingham B. T. Penetration of host cell membranes by adenovirus 2. J Virol. 1973 Aug;12(2):386–396. doi: 10.1128/jvi.12.2.386-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. M., Roos R., Burrell R., Gutmann L., Harley J. B. Subacute focal adenovirus encephalitis. J Neuropathol Exp Neurol. 1973 Jan;32(1):34–50. doi: 10.1097/00005072-197301000-00003. [DOI] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANDSON R. A. A NEW MAGAGLAS, D.E.R.(R) 732, EMBEDMENT FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:704–709. doi: 10.1083/jcb.22.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. K., Bensch K. G., Miller L. R., Hsiung G. D. Microscopy of the release of a simian adenovirus. J Bacteriol. 1965 Dec;90(6):1786–1789. doi: 10.1128/jb.90.6.1786-1789.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashida Y., Yunis E. J. Re-examination of encephalitic brains known to contain intranuclear inclusion bodies: electron-microscopic observations following prolonged fixation in formalin. Am J Clin Pathol. 1970 Apr;53(4):537–543. doi: 10.1093/ajcp/53.4.537. [DOI] [PubMed] [Google Scholar]
- Hoenig E. M., Hirano A., Levine S., Ghatak N. R. The early development and fine structure of allergic adrenalitis. Lab Invest. 1970 Mar;22(3):198–205. [PubMed] [Google Scholar]
- Kalnins V. I., Stich H. F., Yohn D. S. Electron microscopic localization of virus-associated antigens in human amnion cells (AV-3) infected with human adenovirus, type 12. Virology. 1966 Apr;28(4):751–754. doi: 10.1016/0042-6822(66)90259-5. [DOI] [PubMed] [Google Scholar]
- LEDINKO N. Production of noninfectious complement-fixing poliovirus particles in HeLa cells treated with proflavine. Virology. 1958 Oct;6(2):512–524. doi: 10.1016/0042-6822(58)90098-9. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYOR H. D. A LABILE INTRANUCLEAR RNA ASSOCIATED WITH THE DEVELOPMENT OF ADENOVIRUSES. J Exp Med. 1964 Mar 1;119:433–442. doi: 10.1084/jem.119.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., GODMAN G. C., BREITENFELD P. M., ROSE H. M. A correlative study by electron and light microscopy of the development of type 5 adenovirus. I. Electron microscopy. J Exp Med. 1960 Aug 1;112:373–382. doi: 10.1084/jem.112.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis G., Kilham L., Hoenig E. M. Experimental adenovirus infection of the mouse adrenal gland. I. Light microscopic observations. Am J Pathol. 1974 May;75(2):363–374. [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Le Buis J., Bernhard W. Electron microscopy of adenovirus 12 replication. 1. Fine structural changes in the nucleus of infected KB cells. J Virol. 1967 Aug;1(4):817–829. doi: 10.1128/jvi.1.4.817-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Mednis B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J Virol. 1969 Nov;4(5):777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Griffith D., Snitzer J. Fatal pneumonia associated with adenovirus type 7. Am J Dis Child. 1967 Jul;114(1):36–41. doi: 10.1001/archpedi.1967.02090220042007. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. Mitochondrial and nuclear interaction. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):351–352. doi: 10.1083/jcb.2.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton H., Carroll S. Fatal adenovirus pneumonia in infants. Correlation of histologic and electron microscopic observations. Am J Pathol. 1971 Dec;65(3):543–548. [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of the adrenal cortex of the rat under normal and experimental conditions. J Ultrastruct Res. 1971 Jan;34(1):23–71. doi: 10.1016/s0022-5320(71)90004-9. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Roy K. L., Yamamoto T. Macromolecular content of inclusions produced by a canine adenovirus. J Virol. 1972 Oct;10(4):801–809. doi: 10.1128/jvi.10.4.801-809.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. M., Adlersberg L., Hoenig E. M., Ende E., Tchorsch Y. Radiolabeled latex particles ithe investigation of phagocytosis in vivo: clearance curves and histological observations. J Reticuloendothel Soc. 1969 Oct-Dec;6(5):561–589. [PubMed] [Google Scholar]
- Sohier R., Chardonnet Y., Prunieras M. Adenoviruses. Status of current knowledge. Prog Med Virol. 1965;7:253–325. [PubMed] [Google Scholar]
- Sundquist B., Everitt E., Philipson L., Hoglund S. Assembly of adenoviruses. J Virol. 1973 Mar;11(3):449–459. doi: 10.1128/jvi.11.3.449-459.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Stich H. F. Electron microscopy of cells infected with adenovirus type 2. J Virol. 1969 Feb;3(2):198–204. doi: 10.1128/jvi.3.2.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Shahrabadi M. S. Enzyme cytochemistry and autoradiography of adenovirus-infected cells as determined with the electron microscope. Can J Microbiol. 1971 Feb;17(2):249–256. doi: 10.1139/m71-042. [DOI] [PubMed] [Google Scholar]