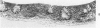Abstract
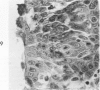
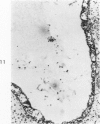
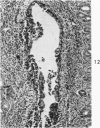
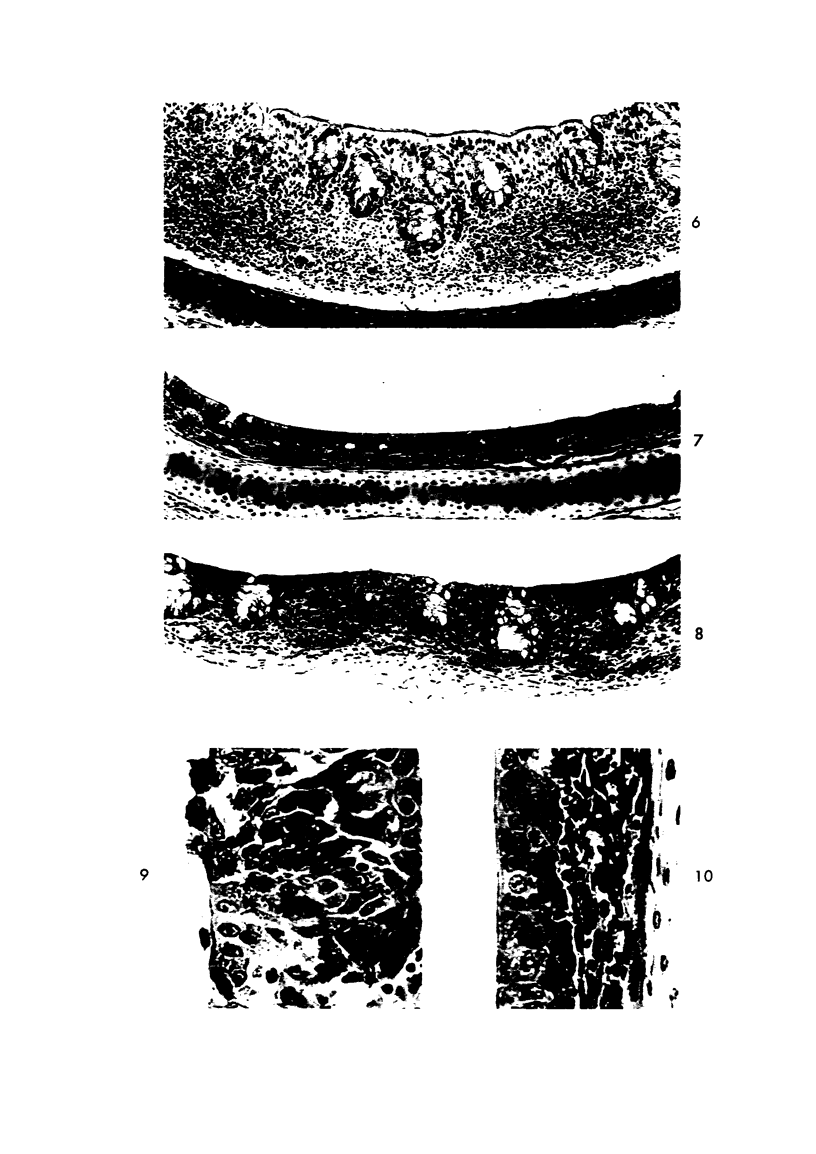
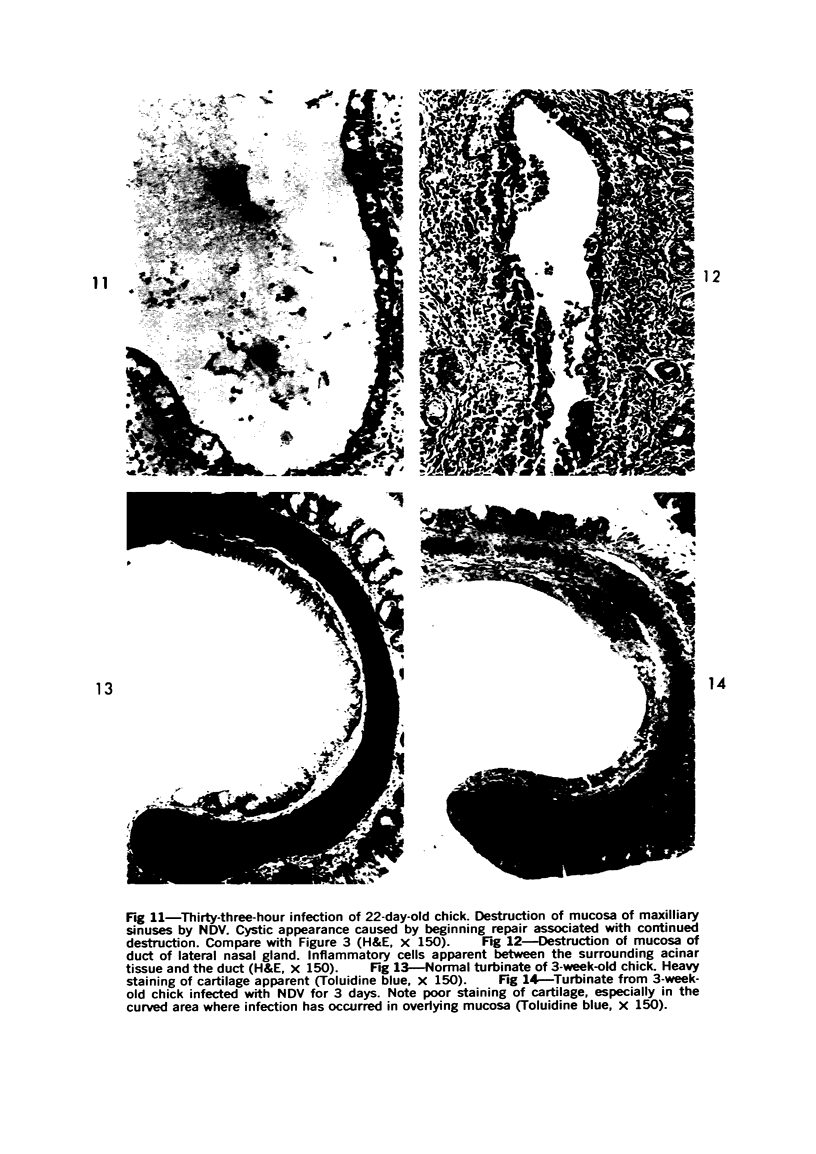
Acute Newcastle disease virus infection following intranasal inoculation of chicks with a mesogenic strain of the virus produced a localized infection of the middle turbinate which was histologically demonstrable 18 hours after inoculation. There was destruction of mucous cells of individual acini in the under surface of the middle turbinate, and the infection rapidly spread to ciliated and goblet cells and to neighboring acini. By day 2 there was simultaneous remodeling of the mucosa, continued destruction and inflammatory infiltration and frequent loss of cartilage basophilia. By day 3 polymorphonuclear cells almost disappeared, epithelial mitoses commenced, and lymphocyte infiltration intensified; the plasma cells normally present along the lateral nasal gland ducts were often destroyed, very occasionally the glands themselves were destroyed. By days 5 and 6 inflammation greatly decreased, and by day 8 the mucociliated epithelium was essentially normal. The infection is sequentially comparable to acute mild rhinitis of man.
Full text
PDF




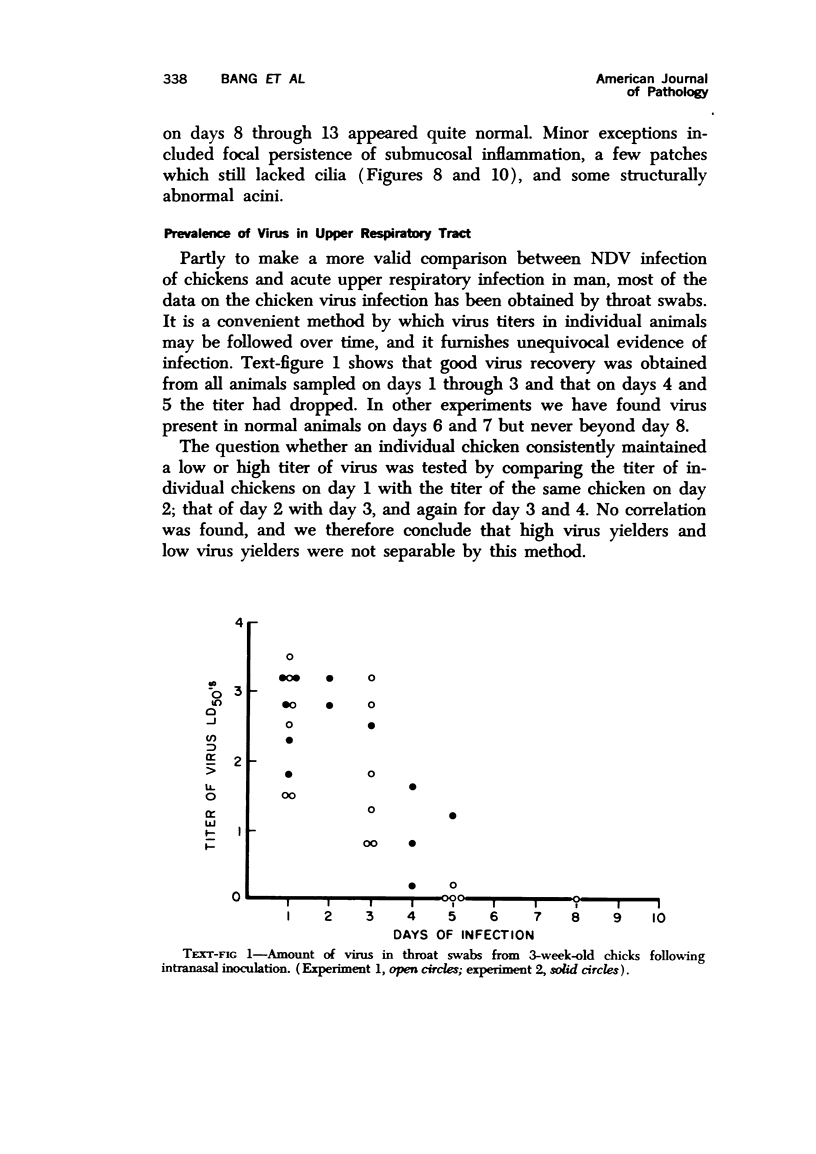






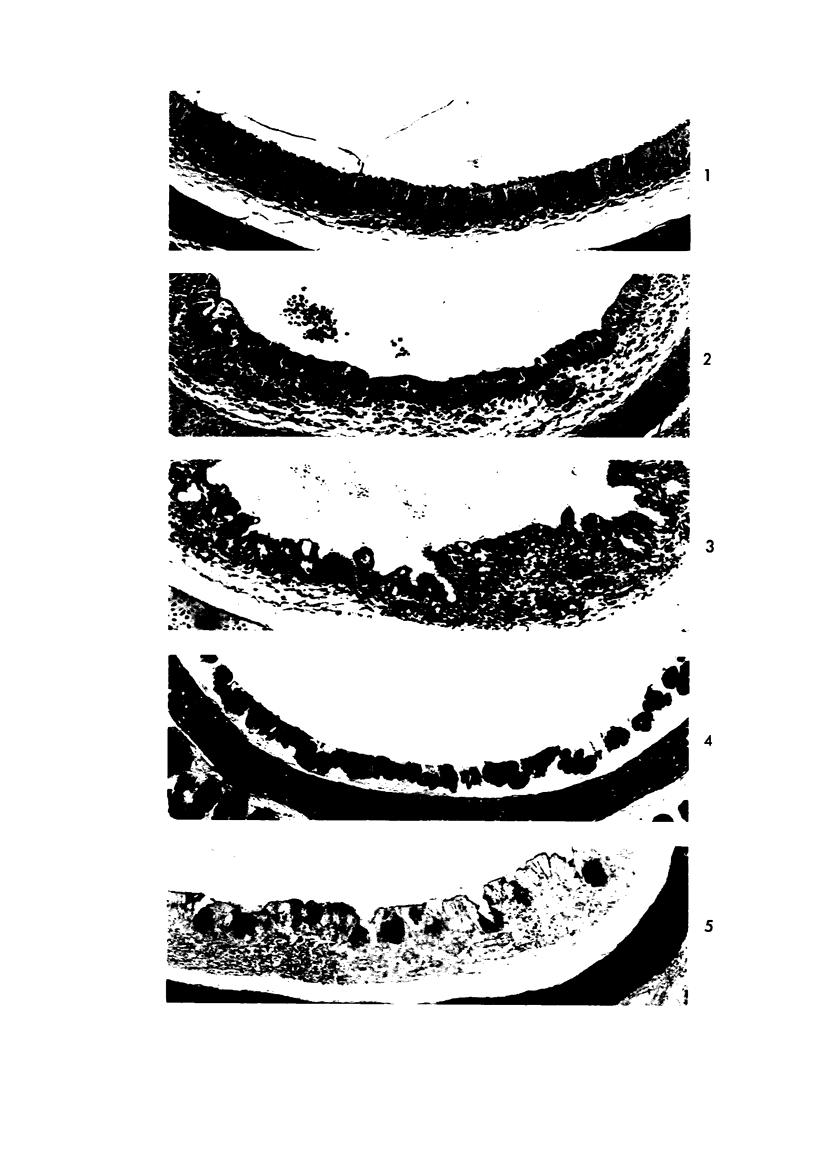

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANG B. G. The surface pattern of the nasal mucosa and its relation to mucous flow--a study of chicken and herring gull nasal mucosae. J Morphol. 1961 Jul;109:57–71. doi: 10.1002/jmor.1051090105. [DOI] [PubMed] [Google Scholar]
- BANG F. B., FOARD M. Flocks of chickens free from antibody to Rous virus. J Natl Cancer Inst. 1963 Mar;30:457–466. [PubMed] [Google Scholar]
- BOJSEN-MOLLER F. GLANDULAE NASALES ANTERIORES IN THE HUMAN NOSE. Ann Otol Rhinol Laryngol. 1965 Jun;74:363–375. doi: 10.1177/000348946507400207. [DOI] [PubMed] [Google Scholar]
- Bang B. G., Bang F. B. Experimentally induced changes in nasal mucous secretory systems and their effect on virus infection in chickens. I. Effect on mucosal mrphology and function. J Exp Med. 1969 Jul 1;130(1):105–119. doi: 10.1084/jem.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang B. G., Bang F. B., Foard M. A. Lymphocyte depression induced in chickens on diets deficient in vitamin A and other components. Am J Pathol. 1972 Jul;68(1):147–162. [PMC free article] [PubMed] [Google Scholar]
- Bang B. G., Bang F. B. Laryngotracheitis virus in chickens. A model for study of acute nonfatal desquamating rhinitis. J Exp Med. 1967 Mar 1;125(3):409–428. doi: 10.1084/jem.125.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang B. G., Bang F. B. Localized lymphoid tissues and plasma cells in paraocular and paranasal organ systems in chickens. Am J Pathol. 1968 Nov;53(5):735–751. [PMC free article] [PubMed] [Google Scholar]
- Bang B. G., Bang F. B. Replacement of virus-destroyed epithelium by keratinized squamous cells in vitamin A-deprived chickens. Proc Soc Exp Biol Med. 1969 Oct;132(1):50–54. doi: 10.3181/00379727-132-34146. [DOI] [PubMed] [Google Scholar]
- Bang F. B., Foard M. A. Experimentally induced changes in nasalucous secretory systems and their effect on virus infection in chickens. II. Effects on adsorption of Newcastle disease virus. J Exp Med. 1969 Jul 1;130(1):121–138. doi: 10.1084/jem.130.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU C., BANG F. B. An analysis of the difference between a destructive and a vaccine strain of NDV (Newcastle disease virus) in the chick embryo. J Immunol. 1953 Jun;70(6):538–548. [PubMed] [Google Scholar]
- Ota W. K., Bang B. G., Bang F. B. Tritiated thymidine labeling of normal and NDV-infected chick nasal epithelial cells. Proc Soc Exp Biol Med. 1971 Sep;137(4):1278–1282. doi: 10.3181/00379727-137-35772. [DOI] [PubMed] [Google Scholar]
- Wight P. A., Burns R. B., Rothwell B., Mackenzie G. M. The Harderian gland of the domestic fowl. I. Histology, with reference to the genesis of plasma cells and Russell bodies. J Anat. 1971 Nov;110(Pt 2):307–315. [PMC free article] [PubMed] [Google Scholar]