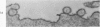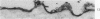Abstract
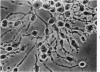
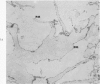
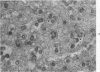
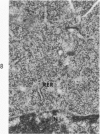
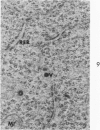
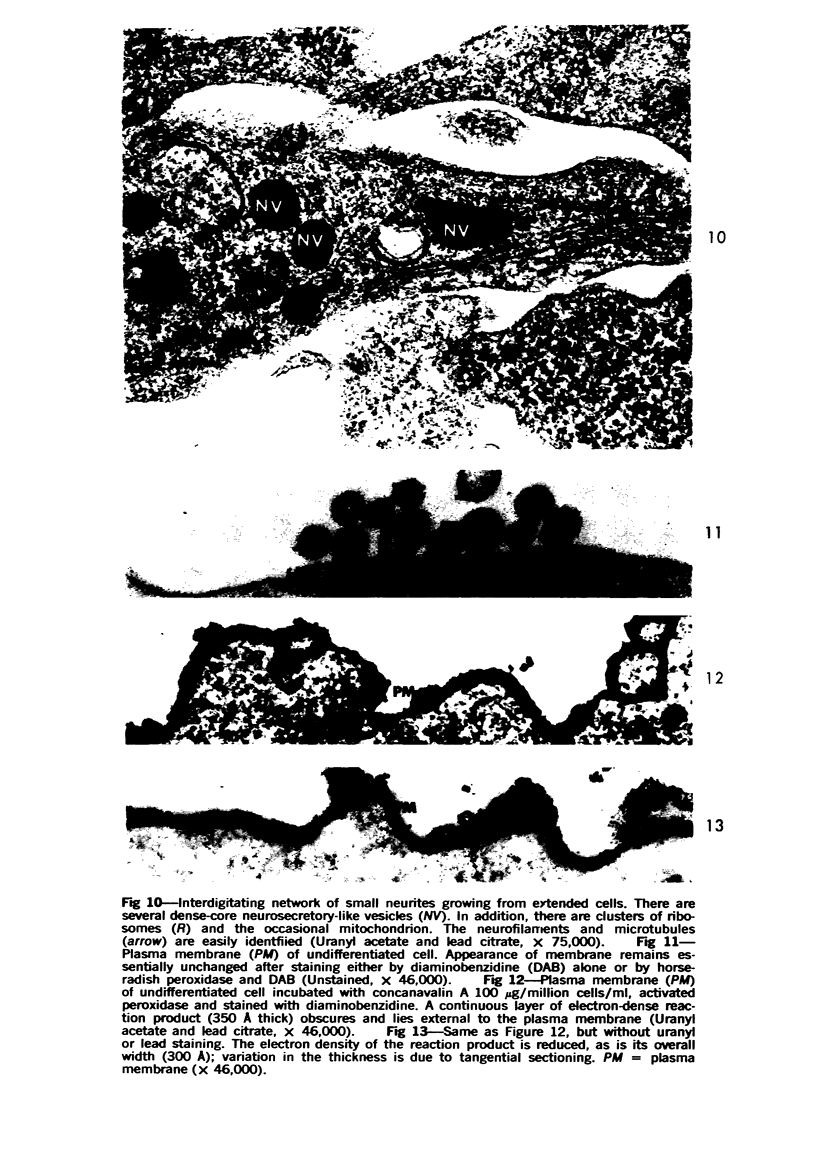
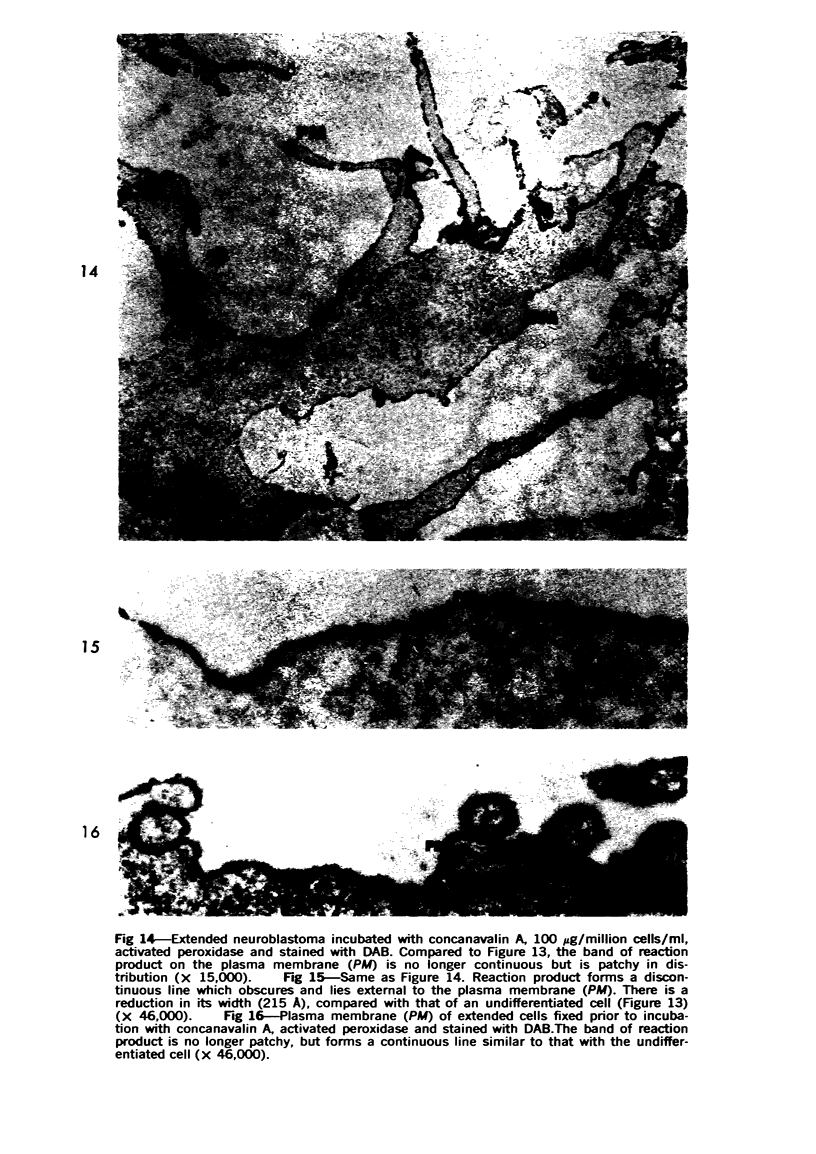
Mouse neuroblastoma cells (clone neuro-2A) in the undifferentiated and “differentiated” form were compared by light and electron microscopy. “Cytodifferentiation” was induced in monolayer cultures by the addition of dibutyryl-cyclic AMP. The pattern of concanavalin A binding sites was studied after coupling with horseradish peroxidase. The following major differences were observed. The differentiated cells are characterized by numerous and long neurites, aggregation of ribosomes into polysomes, an extensive network of neurofilaments and microtubules, many dense-core neurosecretory-like vesicles, a discontinuous pattern of concanavalin A binding sites on the plasma membrane, and an increase of the specific activities of acetylcholinesterase, choline acetylase and tyrosine hydroxylase. In contrast, the undifferentiated cells grown in suspension culture lack neurites, contain dispersed ribosomes, infrequent neurofilaments and microtubules and dense-core neurosecretory-like vesicles, and exhibit a continuous pattern of concanavalin A binding sites. In addition, the specific activities of the above mentioned enzymes are significantly lower.
Full text
PDF





















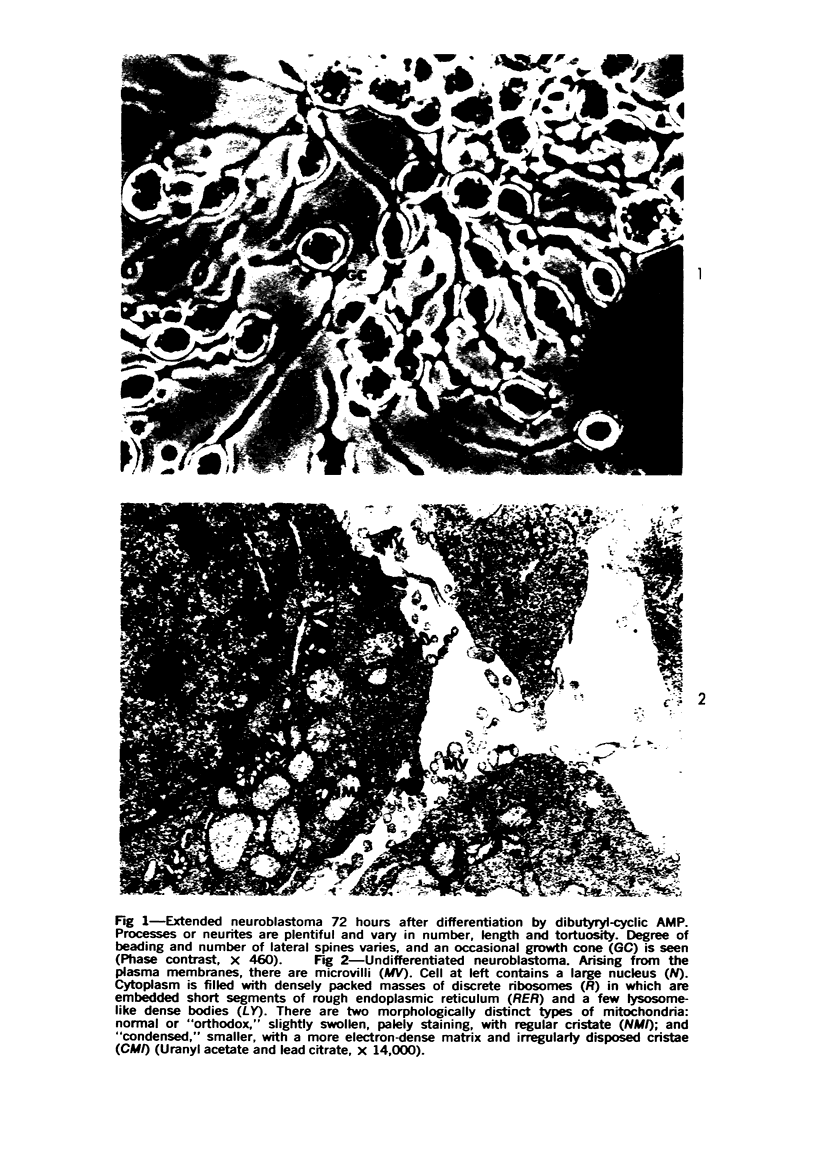
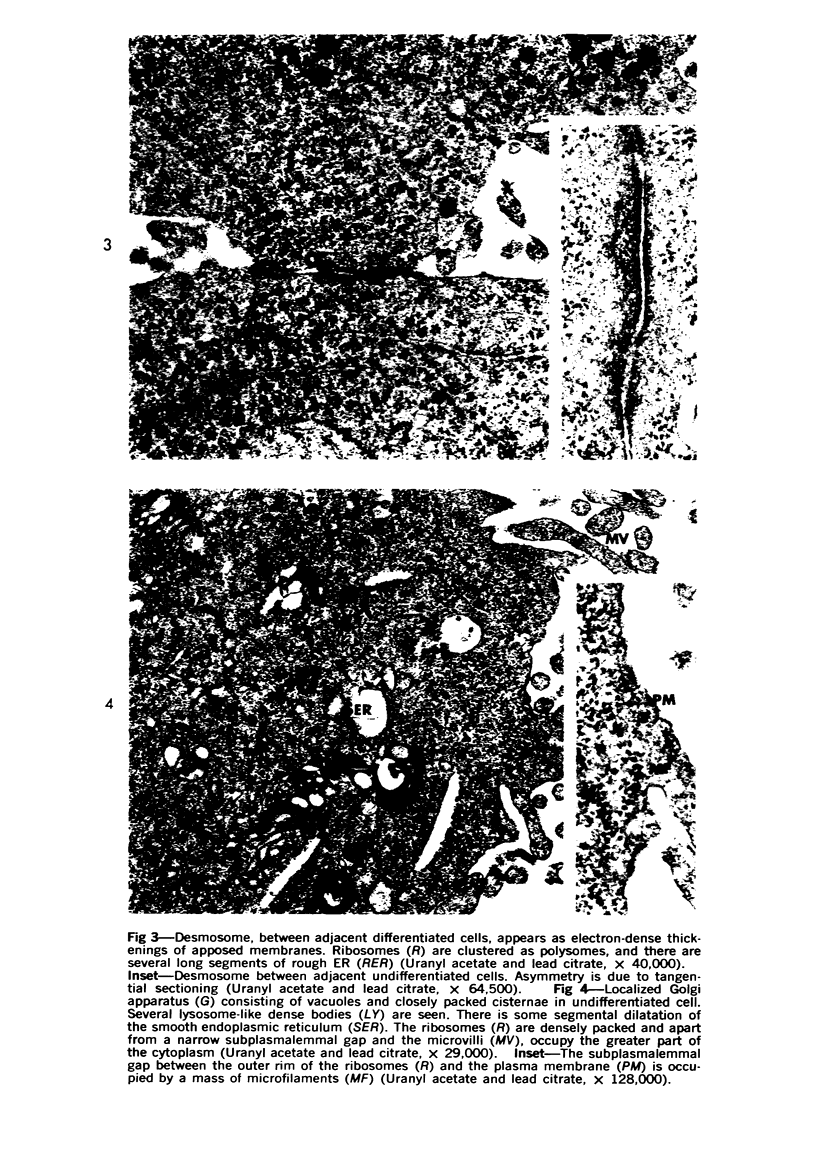
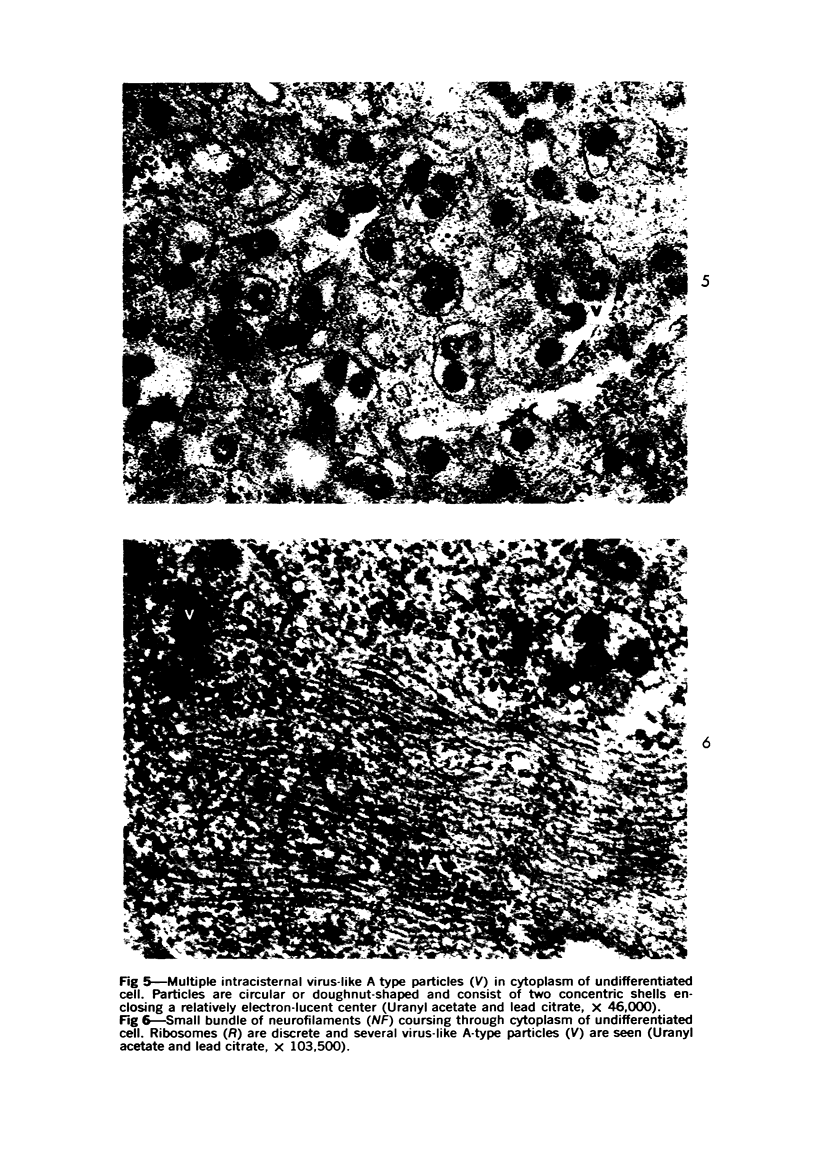

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S. Emploi de la concanavaline-A pour l'isolement, la détection et la mesure des glycoprotéines et glucides extra- ou endo-cellulaires. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 4;18:2205–2208. [PubMed] [Google Scholar]
- Barat N., Avrameas S. Surface and intracellular localization of concanavalin A in human lymphocytes. Exp Cell Res. 1973 Feb;76(2):451–455. doi: 10.1016/0014-4827(73)90401-1. [DOI] [PubMed] [Google Scholar]
- Bernhard W., Avrameas S. Ultrastructural visualization of cellular carbohydrate components by means of concanavalin A. Exp Cell Res. 1971 Jan;64(1):232–236. doi: 10.1016/0014-4827(71)90217-5. [DOI] [PubMed] [Google Scholar]
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretton R., Wicker R., Bernhard W. Ultrastructural localization of concanavalin A receptors in normal and SV 40 -transformed hamster and rat cells. Int J Cancer. 1972 Sep 15;10(2):397–410. doi: 10.1002/ijc.2910100222. [DOI] [PubMed] [Google Scholar]
- Brown J. C. Surface glycoprotein characteristic of the differentiated state of neuroblastoma C-1300 cells. Exp Cell Res. 1971 Dec;69(2):440–442. doi: 10.1016/0014-4827(71)90247-3. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Glycopeptides from the surface of control and virus-transformed cells. Science. 1971 Apr 9;172(3979):169–171. doi: 10.1126/science.172.3979.169. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chang C. M., Goldman R. D. The localization of actin-like fibers in cultured neuroblastoma cells as revealed by heavy meromyosin binding. J Cell Biol. 1973 Jun;57(3):867–874. doi: 10.1083/jcb.57.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S., Raff M. C., Mallucci L. Ligand-induced redistribution of concanavalin A receptors on normal, trypsinized and transformed fibroblasts. Nat New Biol. 1973 Aug 29;244(139):275–278. doi: 10.1038/newbio244275a0. [DOI] [PubMed] [Google Scholar]
- Furmanski P., Silverman D. J., Lubin M. Expression of differentiated functions in mouse neuroblastoma mediated by dibutyryl-cyclic adenosine monophosphate. Nature. 1971 Oct 8;233(5319):413–415. doi: 10.1038/233413a0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Antoine J. C., Stieber A., Avrameas S. Surface immunoglobulins of thymus and lymph node cells demonstrated by the peroxidase coupling technique. Lab Invest. 1972 Mar;26(3):253–261. [PubMed] [Google Scholar]
- Gonatas N. K., Avrameas S. Detection of plasma membrane carbohydrates with lectin peroxidase conjugates. J Cell Biol. 1973 Nov;59(2 Pt 1):436–443. doi: 10.1083/jcb.59.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Greenberg R., Rosenthal I., Falk G. S. Electron microscopyof human tumors secretin catecholamines: correlation with biochemic data. J Neuropathol Exp Neurol. 1969 Jul;28(3):475–500. doi: 10.1097/00005072-196907000-00008. [DOI] [PubMed] [Google Scholar]
- Grillo M. A. Electron microscopy of sympathetic tissues. Pharmacol Rev. 1966 Mar;18(1):387–399. [PubMed] [Google Scholar]
- HUXLEY H. E., ZUBAY G. Preferential staining of nucleic acid-containing structures for electron microscopy. J Biophys Biochem Cytol. 1961 Nov;11:273–296. doi: 10.1083/jcb.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966 Aug;30(2):269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Dennis M. J. Acetylcholine sensitivity and distribution on mouse neuroblastoma cells. Science. 1970 Feb 27;167(3922):1253–1255. doi: 10.1126/science.167.3922.1253. [DOI] [PubMed] [Google Scholar]
- Hier D. B., Arnason B. G., Young M. Studies on the mechanism of action of nerve growth factor. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2268–2272. doi: 10.1073/pnas.69.8.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtnagl H., Winkler H., Asamer H., Födisch H. J., Klima J. Storage of catecholamines in neuroblastoma and ganglioneuroma. A biochemical, immunologic, and morphologic study. Lab Invest. 1972 Dec;27(6):613–619. [PubMed] [Google Scholar]
- Ikeda M., Fahien L. A., Udenfriend S. A kinetic study of bovine adrenal tyrosine hydroxylase. J Biol Chem. 1966 Oct 10;241(19):4452–4456. [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Sachs L. A specific metabolic activity on the surface membrane in malignant cell-transformation. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2748–2751. doi: 10.1073/pnas.68.11.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E., Bensch K. G. Comparison of pheochromocytes with ganglion cells and neuroblasts grown in vitro. An electron microscopic and histochemical study. Cancer. 1971 May;27(5):1148–1160. doi: 10.1002/1097-0142(197105)27:5<1148::aid-cncr2820270521>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kates J. R., Winterton R., Schlessinger K. Induction of acetylcholinesterase activity in mouse neuroblastoma tissue culture cells. Nature. 1971 Jan 29;229(5283):345–347. doi: 10.1038/229345a0. [DOI] [PubMed] [Google Scholar]
- LUSE S. A. SYNAPTIC STRUCTURES OCCURRING IN A NEUROBLASTOMA. Arch Neurol. 1964 Aug;11:185–190. doi: 10.1001/archneur.1964.00460200081007. [DOI] [PubMed] [Google Scholar]
- Martínez-Palomo A. Ultrastructural modifications of intercellular junctions between tumor cells. In Vitro. 1970 Jul-Aug;6(1):15–20. doi: 10.1007/BF02616130. [DOI] [PubMed] [Google Scholar]
- Misugi K., Misugi N., Newton W. A., Jr Fine structural study of neuroblastoma, ganglioneuroblastoma, and pheochromocytoma. Arch Pathol. 1968 Aug;86(2):160–170. [PubMed] [Google Scholar]
- Monard D., Solomon F., Rentsch M., Gysin R. Glia-induced morphological differentiation in neuroblastoma cells. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1894–1897. doi: 10.1073/pnas.70.6.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. D., Meier H., Huebner R. J., Vernon L., Walker J. Cell-free transmission of mouse neuroblastoma. Nature. 1971 Nov 12;234(5324):100–100. doi: 10.1038/234100a0. [DOI] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. A RAPID AND SIMPLE RADIOASSAY FOR TYROSINE HYDROXYLASE ACTIVITY. Anal Biochem. 1964 Sep;9:122–126. doi: 10.1016/0003-2697(64)90092-2. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J. H., Amano T. Responses of neuroblastoma cells to iontophoretically applied acetylcholine. J Cell Physiol. 1971 Jun;77(3):353–362. doi: 10.1002/jcp.1040770309. [DOI] [PubMed] [Google Scholar]
- Nelson P., Ruffner W., Nirenberg M. Neuronal tumor cells with excitable membranes grown in vitro. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1004–1010. doi: 10.1073/pnas.64.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Topography of membrane concanavalin A sites modified by proteolysis. Nat New Biol. 1972 Oct 18;239(94):193–197. doi: 10.1038/newbio239193a0. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B. Peroxisomes in absorptive cells of mammalian small intestine. J Cell Biol. 1972 May;53(2):532–560. doi: 10.1083/jcb.53.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Carlson K., Klebe R., Ruddle F., Rosenbaum J. Isolation of microtubule protein from cultured mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Jan;65(1):129–136. doi: 10.1073/pnas.65.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S. B., Edidin M., Orr C. W., Roseman S. An L-glutamine requirement for intercellular adhesion. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1395–1402. doi: 10.1073/pnas.63.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley R. T., Martin B. J., Spicer S. S. Staining of blood cell surfaces with a lectin-horseradish peroxidase method. J Histochem Cytochem. 1973 Oct;21(10):912–922. doi: 10.1177/21.10.912. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. Morphological differentiation induced by prostaglandin in mouse neuroblastoma cells in culture. Nat New Biol. 1972 Mar 15;236(63):49–52. doi: 10.1038/newbio236049a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Sheppard J. R. Inhibitors of cyclic-nucleotide phosphodiesterase induce morphological differentiation of mouse neuroblastoma cell culture. Exp Cell Res. 1972 Aug;73(2):436–440. doi: 10.1016/0014-4827(72)90069-9. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Vernadakis A. Morphological and biochemical study in x-ray- and dibutyryl cyclic AMP-induced differentiated neuroblastoma cells. Exp Cell Res. 1972 Jan;70(1):27–32. doi: 10.1016/0014-4827(72)90177-2. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. X-ray-induced morphological differentiation of mouse neuroblastoma cells in vitro. Nature. 1971 Dec 24;234(5330):471–473. doi: 10.1038/234471a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Zambernard J., Lasher R., VanWoert M. H. Transmission of mouse neuroblastoma by a cell-free extract. Nature. 1970 Dec 5;228(5275):997–999. doi: 10.1038/228997a0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971 Nov;51(21):536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner M. Serologically demonstrable cell surface specificities on mouse neuroblastoma C1300. Nat New Biol. 1973 May 23;243(125):117–119. [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Jacob F. 5-bromodeoxyuridine-induced differentiation of a neuroblastoma. Proc Natl Acad Sci U S A. 1970 Sep;67(1):247–254. doi: 10.1073/pnas.67.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Shoham J., Sachs L. Differences in the binding of fluorescent concanavalin A to the surface membrane of normal and transformed cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2479–2482. doi: 10.1073/pnas.69.9.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S. Biologically active Concanavalin A complexes suitable for light and electron microscopy. Exp Cell Res. 1972 Feb;70(2):443–447. doi: 10.1016/0014-4827(72)90159-0. [DOI] [PubMed] [Google Scholar]
- Tennyson V. M. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J Cell Biol. 1970 Jan;44(1):62–79. doi: 10.1083/jcb.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waymire J. C., Weiner N., Prasad K. N. Regulation of tyrosine hydroxylase activity in cultured mouse neuroblastoma cells: elevation induced by analogs of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2241–2245. doi: 10.1073/pnas.69.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]