Abstract
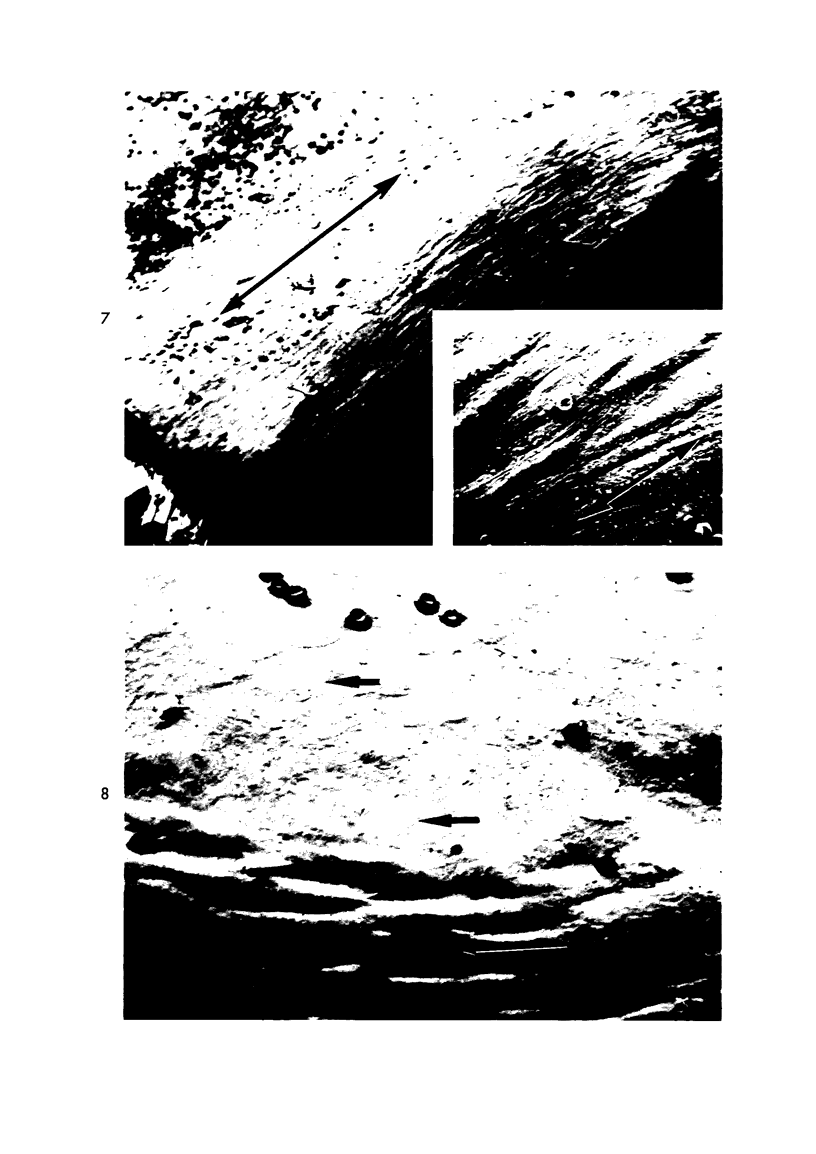
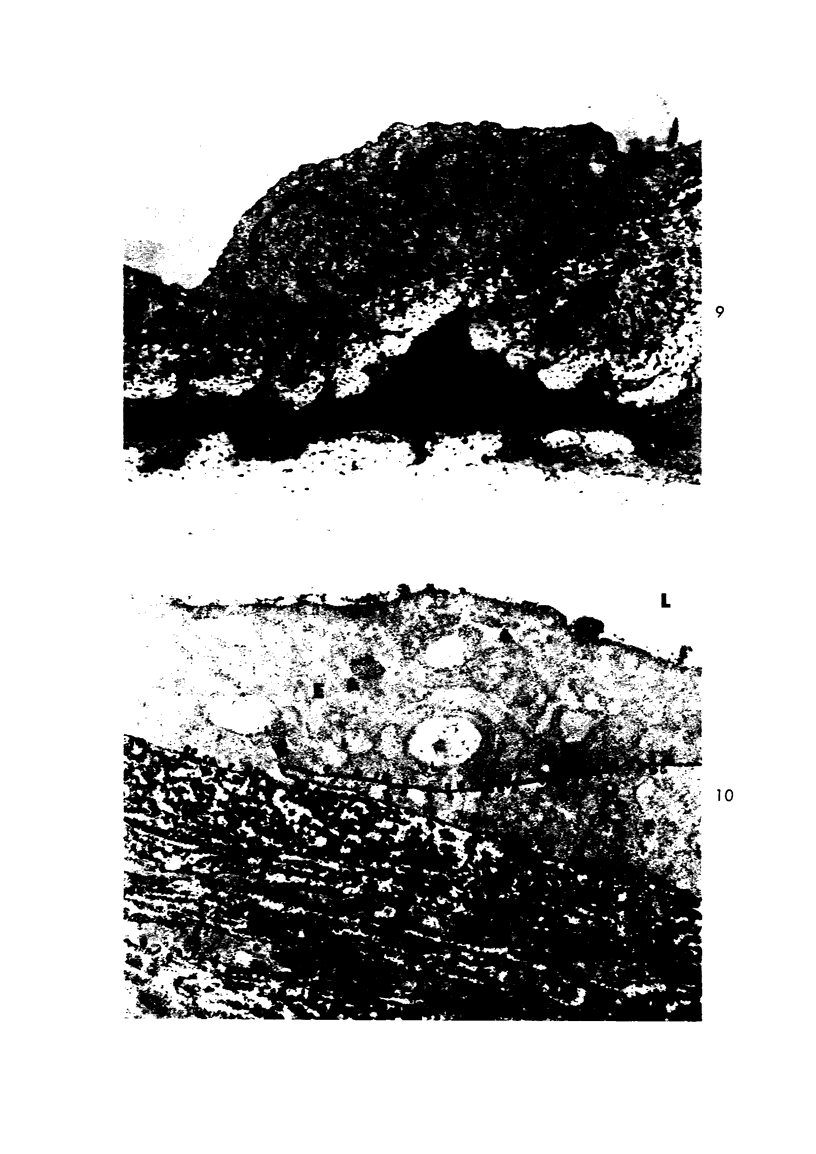
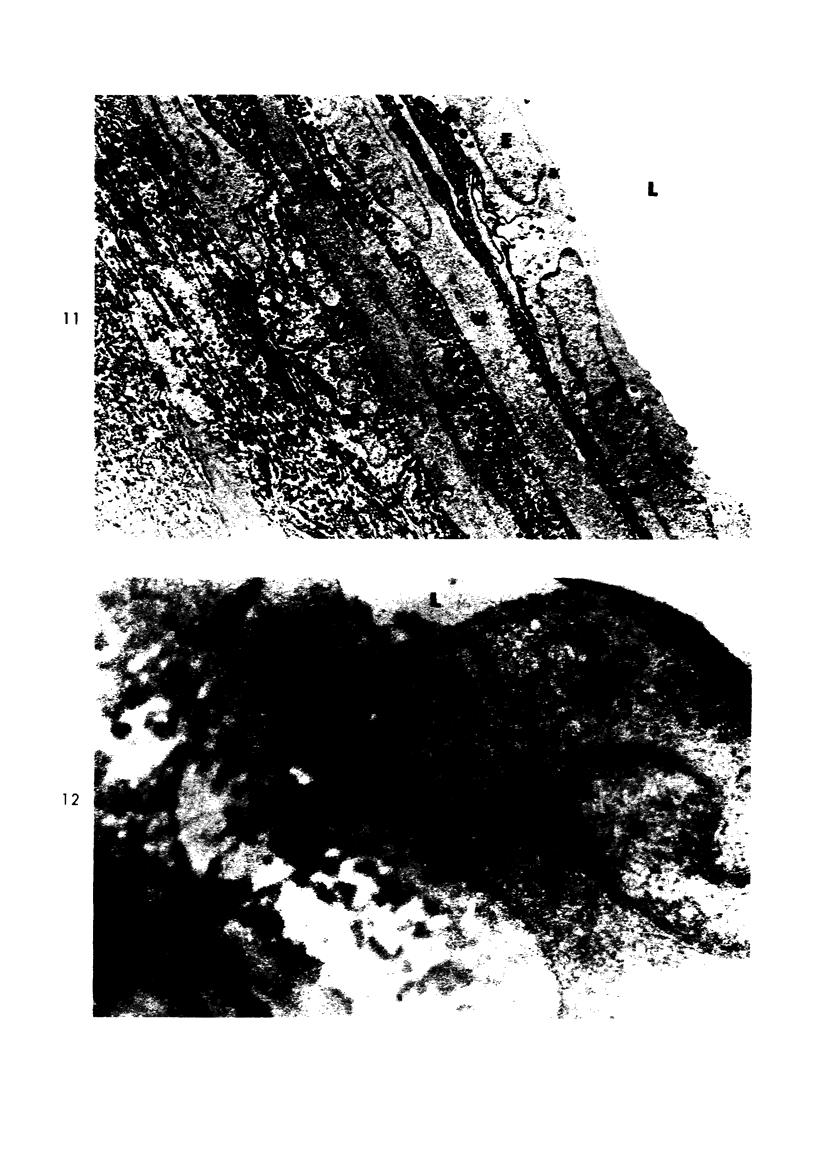
Experimental aortic intimal thickening has been induced in rabbits by two types of injury, suture placement and electrocautery. Scanning electron microscopy showed that endothelialization of the suture plaque was completed at about 10 days following injury. New endothelial cells had no particular orientation or were oriented at right angles to the adjacent normal aortic endothelium. Realignment parallel with the aortic axis had occurred by 21 days after induction of the lesion. Orientation patterns of new endothelial cells over irregularly shaped cautery-induced intimal thickening were difficult to ascertain. Aortic permeability studies were accomplished by using the tracers horseradish peroxidase (HRP) and ferritin. Several naturally occurring intimal thickenings in normal aortas had greater permeability for HRP than did adjacent normal intima. An enhanced penetration of both tracers was observed in mature intimal lesions produced by both experimental procedures compared to adjacent morphologically normal aortic intima. HRP molecules entered the thickened aortic intima in increased amounts through interendothelial junctions and by endothelial pinocytotic vesicles; ferritin molecules were seen only in pinocytotic vesicles. Increased penetration of HRP was observed for as long as 27 weeks after injury, while that of ferritin was observed only for 3 weeks. The enhanced permeability of the thickened intima as compared to normal for these two tracers of considerably different sizes strongly suggests an increased permeability of endothelium overlying intimal thickening for naturally circulating macromolecules.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anversa P., Giacomelli F., Wiener J., Spiro D. Permeability properties of ventricular endocardium. Lab Invest. 1973 Jun;28(6):728–734. [PubMed] [Google Scholar]
- Bruns R. R., Palade G. E. Studies on blood capillaries. II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol. 1968 May;37(2):277–299. doi: 10.1083/jcb.37.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi F., Palade G. E. Intestinal capillaries. I. Permeability to peroxidase and ferritin. J Cell Biol. 1969 Apr;41(1):33–58. doi: 10.1083/jcb.41.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAOUD A., JARMOLYCH J., ZUMBO A., FANI, FLORENTIN R. PREATHEROMA PHASE OF CORONARY ATHEROSCLEROSIS IN MAN. Exp Mol Pathol. 1964 Oct;90:475–484. doi: 10.1016/0014-4800(64)90028-0. [DOI] [PubMed] [Google Scholar]
- DUFF G. L., McMILLAN G. C., LAUTSCH E. V. The uptake of colloidal thorium dioxide by the arterial lesions of cholesterol atherosclerosis in the rabbit; its significance in relation to pathogenesis. Am J Pathol. 1954 Sep-Oct;30(5):941–955. [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961 Nov 1;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty J. T., Pierce J. E., Ferrans V. J., Patel D. J., Tucker W. K., Fry D. L. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res. 1972 Jan;30(1):23–33. doi: 10.1161/01.res.30.1.23. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gross L., Epstein E. Z., Kugel M. A. Histology of the Coronary Arteries and their Branches in the Human Heart. Am J Pathol. 1934 Mar;10(2):253–274.7. [PMC free article] [PubMed] [Google Scholar]
- Hardin N. J., Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. 3. The role of earlier acquired fibromuscular intimal thickening in the pathogenesis of later developing atherosclerosis. Am J Pathol. 1973 Nov;73(2):301–326. [PMC free article] [PubMed] [Google Scholar]
- Hüttner I., Boutet M., More R. H. Studies on protein passage through arterial endothelium. II. Regional differences in permeability to fine structural protein tracers in arterial endothelium of normotensive rat. Lab Invest. 1973 Jun;28(6):678–685. [PubMed] [Google Scholar]
- Hüttner I., More R. H., Rona G. Fine structural evidence of specific mechanism for increased endothelial permeability in experimental hypertension. Am J Pathol. 1970 Dec;61(3):395–412. [PMC free article] [PubMed] [Google Scholar]
- Jensen J. The kinetics of the in vitro cholesterol uptake at the endothelial cell surface of the rabbit aorta. Biochim Biophys Acta. 1967 Jul 3;135(3):544–556. doi: 10.1016/0005-2736(67)90043-0. [DOI] [PubMed] [Google Scholar]
- Lofland H. B., Clarkson T. B. The bi-directional transfer of cholesterol in normal aorta, fatty streaks, and atheromatous plaques. Proc Soc Exp Biol Med. 1970 Jan;133(1):1–8. doi: 10.3181/00379727-133-34394. [DOI] [PubMed] [Google Scholar]
- MOVAT H. Z., MORE R. H., HAUST M. D. The diffuse intimal thickening of the human aorta with aging. Am J Pathol. 1958 Nov-Dec;34(6):1023–1031. [PMC free article] [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. 3. The cholesterol-induced lesions and the effect of a cholesterol and oil diet on the preexisting spontaneous plaque in the chicken aorta. Lab Invest. 1970 Nov;23(5):521–535. [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- PRIOR J. T., JONES D. B. Structural alterations within the aortic intima in infancy and childhood. Am J Pathol. 1952 Sep-Oct;28(5):937–951. [PMC free article] [PubMed] [Google Scholar]
- WESTLAKE G. E., GRUNDY S. M., O'NEAL R. M. THE EFFECT OF AN ATHEROGENIC DIET ON PRE-EXISTING AORTIC INTIMAL THICKENINGS IN OLD DOGS. Exp Mol Pathol. 1963 Aug;52:SUPPL1–SUPPL1:8. [PubMed] [Google Scholar]
- WILENS S. L. The nature of diffuse intimal thickening of arteries. Am J Pathol. 1951 Sep-Oct;27(5):825–839. [PMC free article] [PubMed] [Google Scholar]
- Webster W. S., Bishop S. P., Geer J. C. Experimental aortic intimal thickening. I. Morphology and source of intimal cells. Am J Pathol. 1974 Aug;76(2):245–264. [PMC free article] [PubMed] [Google Scholar]