Abstract
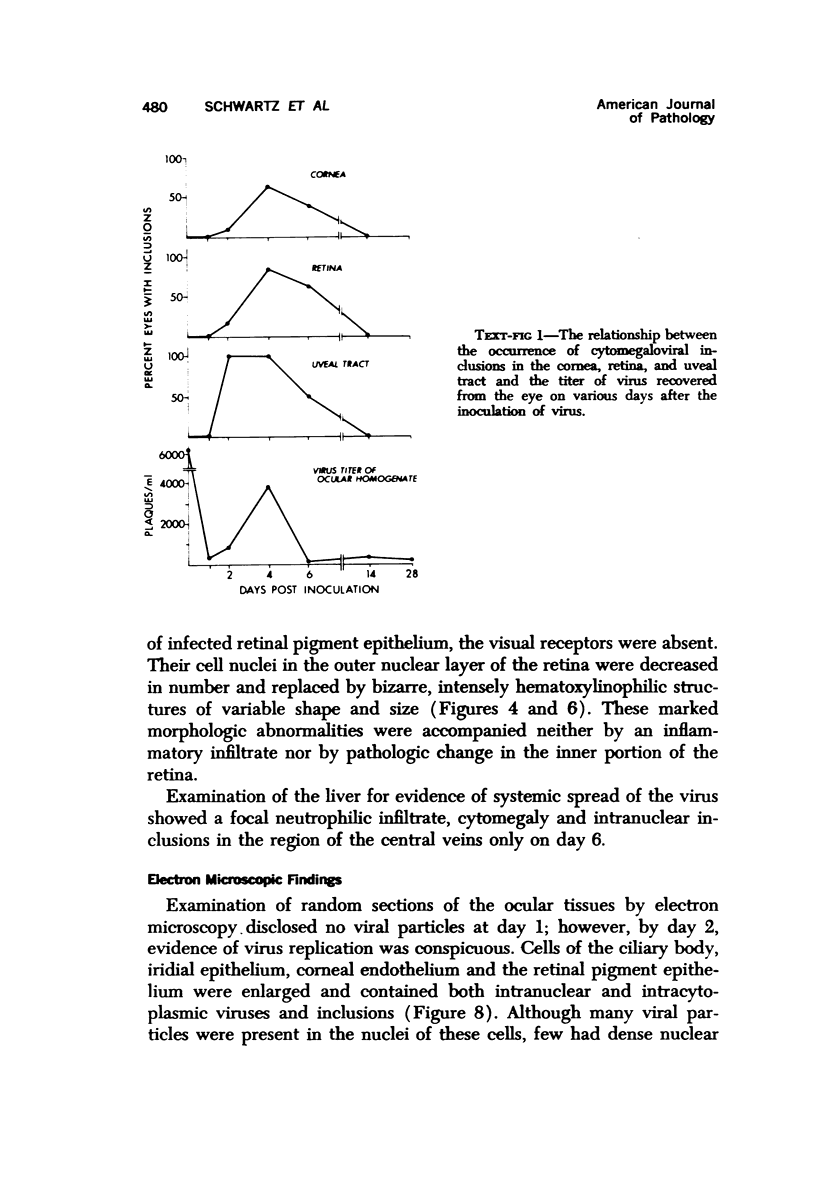
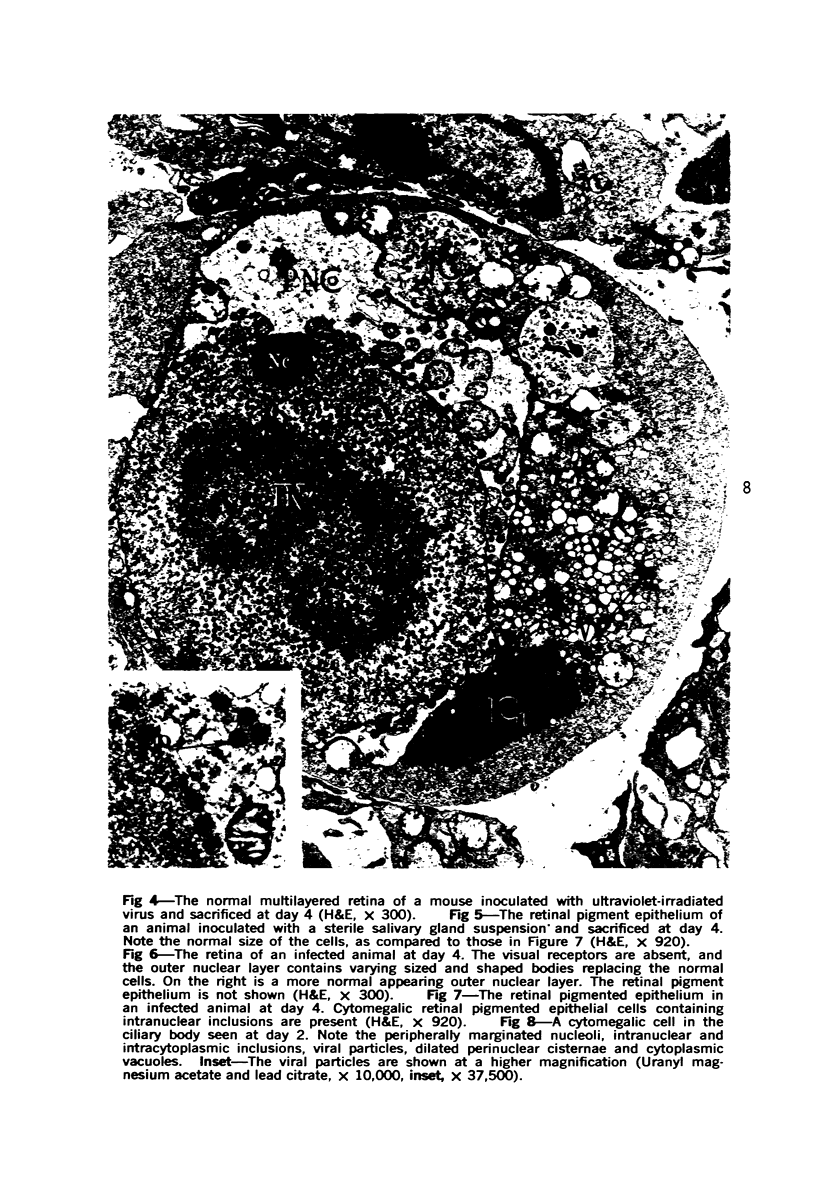
Cytomegalovirus can produce a severe necrotizing chorioretinitis in patients on immunosuppressive therapy and infants born with congenital cytomegaloviral inclusion disease. To study the effect of cytomegalovirus on the eye, murine cytomegalovirus was injected into the eyes of nonimmunosuppressed Swiss CD-1 weanling mice. The eyes were then prepared for virus titer, as well as light and electron microscopy at variable periods after inoculation (1 to 28 days). From days 2 to 6, the hallmarks of cytomegalovirus infection, intranuclear and intracytoplasmic viral inclusions, were evident within cytomegalic cells. The major site of reaction was in the uveal tract, where necrosis and inflammation were prominent. Viral particles budding through the nuclear membranes into the perinuclear cisternae and vacuoles with viral particles could be seen in the cytoplasm of infected cells. In lesions older than 2 weeks, only a mild mixed inflammatory infiltrate and fibrosis were observed. Morphologic alterations unaccompanied by inflammation occurred in the outer sensory retina overlying infected retinal pigment epithelial cells. Multiple necrotic foci with inclusion-bearing cells in the liver indicated the systemic spread of virus from the eye. The titer of virus recovered from the eye peaked at day 4 and then declined to low levels, but infectious virus could still be isolated at day 28, even though viral particles were not seen morphologically at or after day 14. Many of the alterations seen in the model resemble those found in the human cytomegaloviral ophthalmitis.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN A., BEEMAN H. W., CHRISTENSEN L. Cytomegalic inclusion disease. AMA Arch Ophthalmol. 1957 Jan;57(1):90–99. doi: 10.1001/archopht.1957.00930050098018. [DOI] [PubMed] [Google Scholar]
- Aaberg T. M., Cesarz T. J., Rytel M. W. Correlation of virology and clinical course of cytomegalovirus retinitis. Am J Ophthalmol. 1972 Sep;74(3):407–415. doi: 10.1016/0002-9394(72)90899-9. [DOI] [PubMed] [Google Scholar]
- BRODSKY I., ROWE W. P. Chronic subclinical infection with mouse salivary gland virus. Proc Soc Exp Biol Med. 1958 Dec;99(3):654–655. doi: 10.3181/00379727-99-24451. [DOI] [PubMed] [Google Scholar]
- BURNS R. P. Cytomegalic inclusion disease uveitis; report of a case with isolation from aqueous humor of the virus in tissue culture. AMA Arch Ophthalmol. 1959 Mar;61(3):376–387. [PubMed] [Google Scholar]
- Craighead J. E., Kanich R. E., Almeida J. D. Nonviral microbodies with viral antigenicity produced in cytomegalovirus-infected cells. J Virol. 1972 Oct;10(4):766–775. doi: 10.1128/jvi.10.4.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWLING J. E., SIDMAN R. L. Inherited retinal dystrophy in the rat. J Cell Biol. 1962 Jul;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DVORAK-THEOBALD G. Cytomegalic inclusion disease: report of a case. Am J Ophthalmol. 1959 May;47(5 Pt 2):52–56. doi: 10.1016/s0002-9394(14)78228-5. [DOI] [PubMed] [Google Scholar]
- De Venecia G., Zu Rhein G. M., Pratt M. V., Kisken W. Cytomegalic inclusion retinitis in an adult. Arch Ophthalmol. 1971 Jul;86(1):44–57. doi: 10.1001/archopht.1971.01000010046010. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A. Cellular immunity in host resistance to viral infections. Arch Intern Med. 1970 Jul;126(1):125–134. [PubMed] [Google Scholar]
- Henson D., Strano A. J. Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am J Pathol. 1972 Jul;68(1):183–202. [PMC free article] [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. C3 leukotactic factors produced by a tissue protease. J Exp Med. 1969 Sep 1;130(3):505–518. doi: 10.1084/jem.130.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll A. J. Secondary retinal detachment. Electron microscopy of retina and pigment epithelium. Am J Ophthalmol. 1969 Aug;68(2):223–237. [PubMed] [Google Scholar]
- MANSCHOT W. A., DAAMEN C. B. A case of cytomegalic inclusion disease with ocular involvement. Ophthalmologica. 1962;143:137–140. doi: 10.1159/000304210. [DOI] [PubMed] [Google Scholar]
- MIKLOS G., ORBAN T. OPHTHALMIC LESIONS DUE TO CYTOMEGALIC INCLUSION DISEASE. Ophthalmologica. 1964;148:98–106. doi: 10.1159/000304665. [DOI] [PubMed] [Google Scholar]
- Miller J., Howard R. J., Hattler B. G., Najarian J. S. Correlation of MLC reactivity after experimental and clinical transplantation. Virus and cytophilic antibody: sources of false negatives. Transplant Proc. 1973 Dec;5(4):1771–1774. [PubMed] [Google Scholar]
- Osborn J. E., Blazkovec A. A., Walker D. L. Immunosuppression during acute murine cytomegalovirus infection. J Immunol. 1968 Apr;100(4):835–844. [PubMed] [Google Scholar]
- PLUMMER G., BENYESH-MELNICK M. A PLAQUE REDUCTION NEUTRALIZATION TEST FOR HUMAN CYTOMEGALOVIRUS. Proc Soc Exp Biol Med. 1964 Oct;117:145–150. doi: 10.3181/00379727-117-29520. [DOI] [PubMed] [Google Scholar]
- RUEBNER B. H., MIYAI K., SLUSSER R. J., WEDEMEYER P., MEDEARIS D. N., Jr MOUSE CYTOMEGALOVIRUS INFECTION. AN ELECTRON MICROSCOPIC STUDY OF HEPATIC PARENCHYMAL CELLS. Am J Pathol. 1964 May;44:799–821. [PMC free article] [PubMed] [Google Scholar]
- SMITH M. E. RETINAL INVOLVEMENT IN ADULT CYTOMEGALIC INCLUSION DISEASE. Arch Ophthalmol. 1964 Jul;72:44–49. doi: 10.1001/archopht.1964.00970020046011. [DOI] [PubMed] [Google Scholar]
- SMITH M. G. Propagation in tissue cultures of a cytopathogenic virus from human salivary gland virus (SGV) disease. Proc Soc Exp Biol Med. 1956 Jun;92(2):424–430. doi: 10.3181/00379727-92-22498. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Zimmerman L. E., Harley R. D. Ocular involvement in congenital cytomegalic inclusion disease. Arch Ophthalmol. 1966 Nov;76(5):696–699. doi: 10.1001/archopht.1966.03850010698013. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Wohlenberg C., Notkins A. L. Inflammation and viral infection: chemotactic activity resulting from the interaction of antiviral antibody and complement with cells infected with herpes simplex virus. J Infect Dis. 1972 Aug;126(2):207–209. doi: 10.1093/infdis/126.2.207. [DOI] [PubMed] [Google Scholar]
- Tsukahara I., Ueno I., Kawanishi H. Retinal changes in human cytomegalovirus infection. An electron microscopic study. Am J Ophthalmol. 1966 Dec;62(6):1153–1160. doi: 10.1016/0002-9394(66)92569-4. [DOI] [PubMed] [Google Scholar]
- Wyhinny G. J., Apple D. J., Guastella F. R., Vygantas C. M. Adult cytomegalic inclusion retinitis. Am J Ophthalmol. 1973 Nov;76(5):773–781. doi: 10.1016/0002-9394(73)90576-x. [DOI] [PubMed] [Google Scholar]
- YOUNGNER J. S. Monolayer tissue cultures. I. Preparation and standardization of suspensions of trypsin-dispersed monkey kidney cells. Proc Soc Exp Biol Med. 1954 Feb;85(2):202–205. doi: 10.3181/00379727-85-20830. [DOI] [PubMed] [Google Scholar]

















